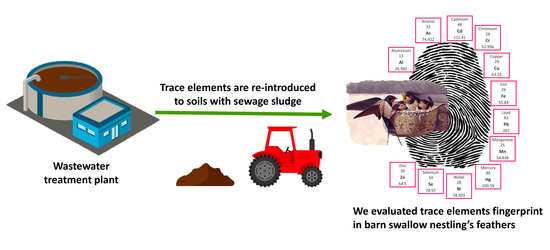
Local Variability of Trace Element Concentration in Barn Swallow (Hirundo rustica) Nestlings from the Po Plain (Northern Italy)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Study Area and Field Methods
2.3. Reagents and Apparatus
2.4. Analytical Procedure
2.5. Statistical Analyses
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Holt, E.A.; Miller, S.W. Bioindicators: Using Organisms to Measure Environmental Impacts|Learn Science at Scitable. Nat. Educ. Knowl. 2011, 3, 8. [Google Scholar]
- Conti, M.E. Environmental Biological Monitoring. Biol. Monit. 2008, 30, 1–23. [Google Scholar] [CrossRef]
- Golden, N.H.; Rattner, B.A. Ranking Terrestrial Vertebrate Species for Utility in Biomonitoring and Vulnerability to Environmental Contaminants. Rev. Environ. Contam. Toxicol. 2003, 176, 67–136. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.N.; Cobb, G.P.; Godard-Codding, C.; Hoff, D.; McMurry, S.T.; Rainwater, T.R.; Reynolds, K.D. Contaminant Exposure in Terrestrial Vertebrates. Environ. Pollut. 2007, 150, 41–64. [Google Scholar] [CrossRef] [PubMed]
- Denneman, W.D.; Douben, P.E.T. Trace Metals in Primary Feathers of the Barn Owl (Tyto alba guttatus) in The Netherlands. Environ. Pollut. 1993, 82, 301–310. [Google Scholar] [CrossRef]
- Bortolotti, G.R. Flaws and Pitfalls in the Chemical Analysis of Feathers: Bad News–Good News for Avian Chemoecology and Toxicology. Ecol. Appl. 2010, 20, 1766–1774. [Google Scholar] [CrossRef]
- Borghesi, F.; Migani, F.; Andreotti, A.; Baccetti, N.; Bianchi, N.; Birke, M.; Dinelli, E. Metals and Trace Elements in Feathers: A Geochemical Approach to Avoid Misinterpretation of Analytical Responses. Sci. Total Environ. 2016, 544, 476–494. [Google Scholar] [CrossRef]
- Parolini, M.; Sturini, M.; Maraschi, F.; Profumo, A.; Costanzo, A.; Caprioli, M.; Rubolini, D.; Ambrosini, R.; Canova, L. Trace Elements Fingerprint of Feathers Differs between Breeding and Non-Breeding Areas in an Afro-Palearctic Migratory Bird, the Barn Swallow (Hirundo rustica). Environ. Sci. Pollut. Res. 2021, 28, 15828–15837. [Google Scholar] [CrossRef]
- Hollamby, S.; Afema-Azikuru, J.; Waigo, S.; Cameron, K.; Rae Gandolf, A.; Norris, A.; Sikarskie, J.G. Suggested Guidelines for Use of Avian Species as Biomonitors. Environ. Monit. Assess. 2006, 118, 13–20. [Google Scholar] [CrossRef]
- Barton, M.G.; Henderson, I.; Border, J.A.; Siriwardena, G. A Review of the Impacts of Air Pollution on Terrestrial Birds. Sci. Total Environ. 2023, 873, 162136. [Google Scholar] [CrossRef]
- Jaspers, V.L.B.; Covaci, A.; Herzke, D.; Eulaers, I.; Eens, M. Bird Feathers as a Biomonitor for Environmental Pollutants: Prospects and Pitfalls. TrAC Trends Anal. Chem. 2019, 118, 223–226. [Google Scholar] [CrossRef]
- Gebbink, W.A.; Letcher, R.J. Comparative Tissue and Body Compartment Accumulation and Maternal Transfer to Eggs of Perfluoroalkyl Sulfonates and Carboxylates in Great Lakes Herring Gulls. Environ. Pollut. 2012, 162, 40–47. [Google Scholar] [CrossRef]
- Sagerup, K.; Savinov, V.; Savinova, T.; Kuklin, V.; Muir, D.C.G.; Gabrielsen, G.W. Persistent Organic Pollutants, Heavy Metals and Parasites in the Glaucous Gull (Larus hyperboreus) on Spitsbergen. Environ. Pollut. 2009, 157, 2282–2290. [Google Scholar] [CrossRef]
- Dauwe, T.; Bervoets, L.; Pinxten, R.; Blust, R.; Eens, M. Variation of Heavy Metals within and among Feathers of Birds of Prey: Effects of Molt and External Contamination. Environ. Pollut. 2003, 124, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Płotka-Wasylka, J.; Lubinska-Szczygeł, M.; Różańska, A.; Możejko-Ciesielska, J.; Namieśnik, J. Birds’ Feathers – Suitable Samples for Determination of Environmental Pollutants. TrAC Trends Anal. Chem. 2018, 109, 97–115. [Google Scholar] [CrossRef]
- Hahn, E.; Hahn, K.; Stoeppler, M. Bird Feathers as Bioindicators in Areas of the German Environmental Specimen Bank—Bioaccumulation of Mercury in Food Chains and Exogenous Deposition of Atmospheric Pollution with Lead and Cadmium. Sci. Total Environ. 1993, 139–140, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.A.; Eeva, T.; Eira, C.; Vaqueiro, J.; Vingada, J.V. Assessing Heavy Metal Pollution Using Great Tits (Parus major): Feathers and Excrements from Nestlings and Adults. Environ. Monit. Assess. 2013, 185, 5339–5344. [Google Scholar] [CrossRef]
- Peterson, S.H.; Ackerman, J.T.; Toney, M.; Herzog, M.P. Mercury Concentrations Vary Within and Among Individual Bird Feathers: A Critical Evaluation and Guidelines for Feather Use in Mercury Monitoring Programs. Environ. Toxicol. Chem. 2019, 38, 1164–1187. [Google Scholar] [CrossRef]
- Burger, M.; Gochfeld, J. Age Differences in Metals in the Blood of Herring (Larus argentatus) and Franklin’s (Larus pipixcan) Gulls. Arch. Environ. Contam. Toxicol. 1997, 33, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Llacuna, S.; Gorriz, A.; Sanpera, C.; Nadal, J. Metal Accumulation in Three Species of Passerine Birds (Emberiza cia, Parus major, and Turdus merula) Subjected to Air Pollution from a Coal-Fired Power Plant. Arch. Environ. Contam. Toxicol. 1995, 28, 298–303. [Google Scholar] [CrossRef]
- Eens, M.; Pinxten, R.; Verheyen, R.F.; Blust, R.; Bervoets, L. Great and Blue Tits as Indicators of Heavy Metal Contamination in Terrestrial Ecosystems. Ecotoxicol. Environ. Saf. 1999, 44, 81–85. [Google Scholar] [CrossRef]
- Roig, N.; Sierra, J.; Martí, E.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Long-Term Amendment of Spanish Soils with Sewage Sludge: Effects on Soil Functioning. Agric. Ecosyst. Environ. 2012, 158, 41–48. [Google Scholar] [CrossRef]
- Manzetti, S.; van der Spoel, D. Impact of Sludge Deposition on Biodiversity. Ecotoxicology 2015, 24, 1799–1814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, A.Z.; Cen, T.; Li, X.; He, M.; Li, D.; Chen, J. Sub-Inhibitory Concentrations of Heavy Metals Facilitate the Horizontal Transfer of Plasmid-Mediated Antibiotic Resistance Genes in Water Environment. Environ. Pollut. 2018, 237, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Seshadri, B.; Bolan, N.; Sarkar, B.; Ok, Y.S.; Zhang, W.; Rumpel, C.; Sparks, D.; Farrell, M.; Hall, T.; et al. Microbial Functional Diversity and Carbon Use Feedback in Soils as Affected by Heavy Metals. Environ. Int. 2019, 125, 478–488. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals. In Heavy Metals; Saleh, H.E.-D.M., Aglan, R.F., Eds.; IntechOpen: london, UK, 2018; pp. 115–132. [Google Scholar]
- Donatello, S.; Cheeseman, C.R. Recycling and Recovery Routes for Incinerated Sewage Sludge Ash (ISSA): A Review. Waste Manag. 2013, 33, 2328–2340. [Google Scholar] [CrossRef] [PubMed]
- Kirchmann, H.; Börjesson, G.; Kätterer, T.; Cohen, Y. From Agricultural Use of Sewage Sludge to Nutrient Extraction: A Soil Science Outlook. Ambio 2017, 46, 143–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grobelak, A.; Grosser, A.; Kacprzak, M.; Kamizela, T. Sewage Sludge Processing and Management in Small and Medium-Sized Municipal Wastewater Treatment Plant-New Technical Solution. J. Environ. Manag. 2019, 234, 90–96. [Google Scholar] [CrossRef]
- Grobelak, A.; Czerwińska, K.; Murtaś, A. General Considerations on Sludge Disposal, Industrial and Municipal Sludge. In Industrial and Municipal Sludge; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–153. [Google Scholar]
- Rorat, A.; Courtois, P.; Vandenbulcke, F.; Lemiere, S. Sanitary and Environmental Aspects of Sewage Sludge Management. In Industrial and Municipal Sludge; Elsevier: Amsterdam, The Netherlands, 2019; pp. 155–180. [Google Scholar]
- Bandini, F.; Taskin, E.; Vaccari, F.; Soldano, M.; Piccinini, S.; Frache, A.; Remelli, S.; Menta, C.; Sandro Cocconcelli, P.; Puglisi, E. Anaerobic Digestion and Aerobic Composting of Rigid Biopolymers in Bio-Waste Treatment: Fate and Effects on the Final Compost. Bioresour. Technol. 2022, 351, 126934. [Google Scholar] [CrossRef]
- Wear, S.L.; Acuña, V.; McDonald, R.; Font, C. Sewage Pollution, Declining Ecosystem Health, and Cross-Sector Collaboration. Biol. Conserv. 2021, 255, 109010. [Google Scholar] [CrossRef]
- Ekane, N.; Barquet, K.; Rosemarin, A. Resources and Risks: Perceptions on the Application of Sewage Sludge on Agricultural Land in Sweden, a Case Study. Front. Sustain. Food Syst. 2021, 5, 647780. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging Pollutants in Wastewater: A Review of the Literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Cantinho, P.; Matos, M.; Trancoso, M.A.; dos Santos, M.M.C. Behaviour and Fate of Metals in Urban Wastewater Treatment Plants: A Review. Int. J. Environ. Sci. Technol. 2016, 13, 359–386. [Google Scholar] [CrossRef] [Green Version]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The Fate of Microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef]
- Plumlee, M.H.; Gurr, C.J.; Reinhard, M. Recycled Water for Stream Flow Augmentation: Benefits, Challenges, and the Presence of Wastewater-Derived Organic Compounds. Sci. Total Environ. 2012, 438, 541–548. [Google Scholar] [CrossRef]
- Tang, J.; Tang, H.; Sima, W.; Qiu, B.; Zou, D.; Liang, R.; Dong, J.; Liang, C.; Wan, S.; Li, Z.; et al. Physicochemical Properties and Heavy Metal Pollution Characteristics and Correlation Analysis of Sludge Landfill. Int. J. Environ. Anal. Chem. 2021, 1–10. [Google Scholar] [CrossRef]
- Washburn, B.E.; Begier, M.J. Wildlife Responses to Long-Term Application of Biosolids to Grasslands in North Carolina. Rangel. Ecol. Manag. 2011, 64, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Lovett, R.A. How Birds Are Used to Monitor Pollution. Nature 2012. [Google Scholar] [CrossRef]
- Szép, T.; Hobson, K.A.; Vallner, J.; Piper, S.E.; Kovács, B.; Szabó, D.Z.; Møller, A.P. Comparison of Trace Element and Stable Isotope Approaches to the Study of Migratory Connectivity: An Example Using Two Hirundine Species Breeding in Europe and Wintering in Africa. J. Ornithol. 2009, 150, 621–636. [Google Scholar] [CrossRef]
- Ambrosini, R.; Bolzern, A.M.; Canova, L.; Arieni, S.; Møller, A.P.; Saino, N. The Distribution and Colony Size of Barn Swallows in Relation to Agricultural Land Use. J. Appl. Ecol. 2002, 39, 524–534. [Google Scholar] [CrossRef]
- Liechti, F.; Scandolara, C.; Rubolini, D.; Ambrosini, R.; Korner-Nievergelt, F.; Hahn, S.; Lardelli, R.; Romano, M.; Caprioli, M.; Romano, A.; et al. Timing of Migration and Residence Areas during the Non-Breeding Period of Barn Swallows Hirundo rustica in Relation to Sex and Population. J. Avian Biol. 2015, 46, 254–265. [Google Scholar] [CrossRef]
- Pancerasa, M.; Ambrosini, R.; Romano, A.; Rubolini, D.; Winkler, D.W.; Casagrandi, R. Across the Deserts and Sea: Inter-Individual Variation in Migration Routes of South-Central European Barn Swallows (Hirundo rustica). Mov. Ecol. 2022, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Grigolo, C.P.; Sicurella, B.; Musitelli, F.; Romano, A.; Caprioli, M.; Rubolini, D.; Ambrosini, R.; Gobbi, M. Diet Heteogeneity and Antioxidant Defence in Barn Swallow Hirundo rustica Nestlings. Avocetta 2019, 43, 127–137. [Google Scholar]
- van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: An Introduction to Ordination. Management 2019, 1, 1–10. [Google Scholar]
- Brooks, M.; Bolker, B.; Kristensen, K.; Maechler, M. Package ‘GlmmTMB’. 2022. Available online: https://cran.r-project.org/web/packages/glmmTMB/glmmTMB.pdf (accessed on 1 April 2023).
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version. 2018. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 1 April 2023).
- Mantovani, I. Cartografia Geochimica della Pianura Padana Centrale a Ovest di Mantova. Master’s Thesis, University of Bologna, Bologna, Italy, 2014. [Google Scholar]
- Amorosi, A.; Sammartino, I. Influence of sediment provenance on background values of potentially toxic metals from near-surface sediments of Po coastal plain (Italy). Int. J. Earth Sci. 2007, 96, 389–396. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Metal Levels in Feathers of 12 Species of Seabirds from Midway Atoll in the Northern Pacific Ocean. Sci. Total Environ. 2000, 257, 37–52. [Google Scholar] [CrossRef]
- Pedersen, H.C.; Saether, M. Effects of Cadmium on Parental Behaviour in Free-Living Willow Ptarmigan hens. Ecotoxicology 1999, 8, 1–7. [Google Scholar] [CrossRef]
- Pokras, M.A.; Kneeland, M.R. Lead Poisoning: Using Transdisciplinary Approaches to Solve an Ancient Problem. Ecohealth 2008, 5, 379–385. [Google Scholar] [CrossRef]
- Heinz, G.H.; Hoffman, D.J.; Klimstra, J.D.; Stebbins, K.R.; Kondrad, S.L.; Erwin, C.A. Species Differences in the Sensitivity of Avian Embryos to Methylmercury. Arch. Environ. Contam. Toxicol. 2009, 56, 129–138. [Google Scholar] [CrossRef]
- Tan, S.W.; Meiller, J.C.; Mahaffey, K.R. The Endocrine Effects of Mercury in Humans and Wildlife. Crit. Rev. Toxicol. 2009, 39, 228–269. [Google Scholar] [CrossRef] [PubMed]
- Tartu, S.; Goutte, A.; Bustamante, P.; Angelier, F.; Moe, B.; Clément-Chastel, C.; Bech, C.; Gabrielsen, G.W.; Bustnes, J.O.; Chastel, O. To Breed or Not to Breed: Endocrine Response to Mercury Contamination by an Arctic Seabird. Biol. Lett. 2013, 9, 20130317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goutte, A.; Barbraud, C.; Meillère, A.; Carravieri, A.; Bustamante, P.; Labadie, P.; Budzinski, H.; Delord, K.; Cherel, Y.; Weimerskirch, H.; et al. Demographic Consequences of Heavy Metals and Persistent Organic Pollutants in a Vulnerable Long-Lived Bird, the Wandering Albatross. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133313. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costanzo, A.; Sturini, M.; Maraschi, F.; Caprioli, M.; Romano, A.; Vanni, S.; Parolini, M.; Profumo, A.; Rubolini, D.; Ambrosini, R.; et al. Local Variability of Trace Element Concentration in Barn Swallow (Hirundo rustica) Nestlings from the Po Plain (Northern Italy). Environments 2023, 10, 145. https://doi.org/10.3390/environments10080145
Costanzo A, Sturini M, Maraschi F, Caprioli M, Romano A, Vanni S, Parolini M, Profumo A, Rubolini D, Ambrosini R, et al. Local Variability of Trace Element Concentration in Barn Swallow (Hirundo rustica) Nestlings from the Po Plain (Northern Italy). Environments. 2023; 10(8):145. https://doi.org/10.3390/environments10080145
Chicago/Turabian StyleCostanzo, Alessandra, Michela Sturini, Federica Maraschi, Manuela Caprioli, Andrea Romano, Simone Vanni, Marco Parolini, Antonella Profumo, Diego Rubolini, Roberto Ambrosini, and et al. 2023. "Local Variability of Trace Element Concentration in Barn Swallow (Hirundo rustica) Nestlings from the Po Plain (Northern Italy)" Environments 10, no. 8: 145. https://doi.org/10.3390/environments10080145
APA StyleCostanzo, A., Sturini, M., Maraschi, F., Caprioli, M., Romano, A., Vanni, S., Parolini, M., Profumo, A., Rubolini, D., Ambrosini, R., & Canova, L. (2023). Local Variability of Trace Element Concentration in Barn Swallow (Hirundo rustica) Nestlings from the Po Plain (Northern Italy). Environments, 10(8), 145. https://doi.org/10.3390/environments10080145