The Impact of Micro- and Nanoplastics on Aquatic Organisms: Mechanisms of Oxidative Stress and Implications for Human Health—A Review
Abstract
:1. Pollution and the Environment
2. Micro- and Nanoplastics
3. Characteristics and Sources
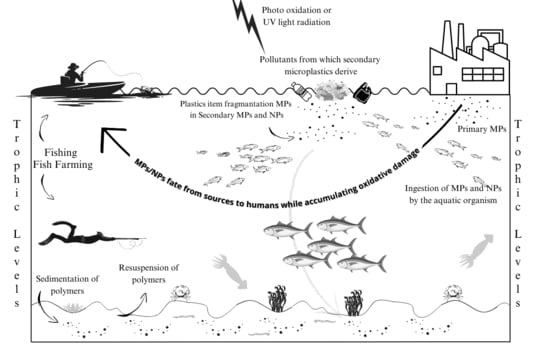
4. Environmental Life-Cycle of Plastic Debris
5. Reactive Oxygen Species and Micro- and Nanoplastics
6. The Impact of Micro- and Nanoplastics on the Aquatic Environment
6.1. MPs/NPs’ Effects on Phytoplankton
6.2. MPs/NPs in Sediments and Benthos
6.3. Trophic Levels: From Zooplankton to Main Fishes Consumed by Humans
6.3.1. MPs/NPs’ Effects on Zooplankton
6.3.2. MPs/NPs’ Effects on Others Aquatic Trophic Levels
6.4. MPs/NPs’ Effects on the Redox Homeostasis of the Aquatic Organisms
6.4.1. NPs
| Redox Homeostasis in Danio Rerio Exposed to PS-NPs | ||||||
|---|---|---|---|---|---|---|
| Author | Age | Exposition | Tissue | Results | ||
| Size | Concentration | Time | ||||
| Bhagat et al. [174] | 96 hpf | 50 nm | 1 mgL−1 | ~2–96 hpf | / | ↑ROS ↑CAT ↓SOD ↓GR |
| Sendra et al. [175] | 96 hpf | 50 nm, 1 µm | 10 mgL−1 | 24 h | / | ↑ROS |
| Pitt et al. [177] | 96 hpf | 42 nm | 10% add. to diet | 1 week | Co-parental | ↓Thiols ↓GR |
| Sarasamma et al. [176] | Adult | 70 nm | 0.5 ppm, 1.5 ppm | 1 week | Muscle Liver | ↑ROS NS |
| Pitt et al. [177] | Adult | 42 nm | 10% add. to diet | 1 week | Male muscle | ↓GR |
| Aliakbarzadeh et al. [178] | Adult | 20–80 nm | 0.1 µgL−1, 1 gL−1, 10 gL−1, 100 gL−1 | 45 days | Gut | ↓CAT ↓t-GSH |
| Umamaheswari et al. [179] | Adult | 103–113 nm | 5 mgL−1, 10 mgL−1 | 14 days | Gills | ↓SOD (5 mgL−1) ↓TAC (10 mgL−1) |
| Lu et al. [180] | Adult | 70 nm | 20 µgL−1, 200 µgL−1, 2 mgL−1 | 3 weeks | Liver | ↑SOD (2 mgL−1) ↑CAT (2 mgL−1) |
6.4.2. MPs
| Redox Homeostasis in Danio Rerio Exposed to PS-MPs | ||||||
|---|---|---|---|---|---|---|
| Author | Age | Exposition | Tissue | Results | ||
| Size | Concentration | Time | ||||
| Santos et al. [181] | 96 hpf | 1,5 µm | 2 mgL−1 | / | / | NS |
| Yang et al. [182] | 6 dpf | 5 μm | 50 ngmL−1 | 1 week | / | NS |
| Chen et al. [183] | 30 dpf | 5 μm | 500 μgL−1 | 25 days | / | ↓CAT |
| Guimarães et al. [173] | Juvenile | ~17 µm | 4 × 104, 4 × 106 particles/m3 | 5 days | / | ↑TBARS ↑CAT ↑SOD ↑t-GSH |
| Qiao et al. [184] | Adult | 5 µm | 50 μgL−1, 500 μgL−1 | 21 days | Gut | ↑CAT ↑SOD |
| Li et al. [185] | Adult | 9, 10 µm | 10 mgL−1 | 4 days 8 days | Liver | 4 d ↑SOD ↑CAT ↑GPX NS MDA 8 d NS SOD ↑CAT ↑GPX ↓MDA |
| Lu et al. [180] | Adult | 5 µm | 20 μgL−1, 200 μgL−1, 2 mgL−1 | 3 weeks | Liver | ↑SOD ↑CAT (200 μgL−1, 2 mgL−1) |
| Authors | Species | Type of MPs/NPs | Exposition Time | Effects |
|---|---|---|---|---|
| Khatiwada et al. [118] | Scenedesmus sp. | Micro-PET | 24 days | Negative influence on the growth of microalgae and eventually reducing chlorophyll. |
| Zhang et al. [119] | Skeletonema costatum | Micro-PVC | 96 h | Growth inhibition and chlorophyll. |
| Guschina et al. [120] | Chlorella sorokiniana | Micro-PS <70 μm | 4 weeks | Alteration of the concentrations of fatty acid molecules and lipid synthesis. |
| Cole et al. [123] | Mytilus edulis | Micro-PS 20 μm/Microfibers-PA 10 × 30 μm/Nano-PS 50 nm | 24 h 7 days | Higher SOD activity after 24 h exposure. SOD activity returned to normal values after 7 days. Nanoplastics increased the proportion of phagocytic hemocytes and resulted in a marked increase in micronuclei formation. |
| Sussarellu et al. [125] | Crassostrea gigas | Micro-PS 2–6 µm | 2 months | Exposed oysters, hyalinocytes, and granulocytes > control. Exposed oocytes and oocyte diameter < control. Exposed sperm velocity < control. Exposed Progeny size and growth < control. |
| Murray and Cowie et al. [126] | Nephrops norvegicus | PP | 24 h each tank five times 2 weeks total | Plastic in stomachs; potential implications for human health. |
| Cole et al. [130] | Centropages typicus | Micro-PS 7.3 μm | 24 h | Decreased algal feeding and negative impact upon health and zooplankton function. |
| Ziajahromi et al. [131] | Ceriodaphnia dubia | Micro-PS/PE 1–4 μm | 48 h 8 days | 50% reduction in reproductive and malformations in the carapace. |
| Rehse et al. [132] | Daphnia magna | Micro-PE 1 μm | 96 h | Immobilization and EC50 of 1-μm was 57.43 mgL−1 after 96 h. |
| Cole et al. [133] | Calanus helgolandicus | Micro-PS 20 μm | 24 h 9 days | Decrease in microalgae ingestion and reduction in egg size. |
| Redondo-Hasselerharm et al. [148] | Gammarus Pulex | Micro-PS 20–500 μm | 28 days | Growth decreased. |
| Straub et al. [149] | Gammarus Fossarum | Micro-PHB PMMA 32–250 μm | 28 days | Growth decreased. |
| Kardgar et al. [150] | Perca fluviatilis | PLA 90–150 μm | Over 6 months | Reduced growth; hindered hatching; and impaired their performance, nutrition, and behavior. |
| Abihssira-García et al. [159] | Salmo salar | Micro PS/PE 1–5 μm | 1, 24, 48, and 72 h | Immune cells can phagocytose MPs and the mortality of the blood cells, distal intestine, and head kidney is affected by the time exposure, and the impact is dependent on the microplastic type. |
| Lu et al. [161] | Danio Rerio | Micro-PS 5 μm–70 nm | 1 week | Increased inflammation, oxidative stress, altered metabolic liver profile. |
| Jeong et al. [162] | Brachionus koreanus | Micro-PS 0.05–0.5–6 μm | 12 days | Increased oxidative stress and decreased growth rate, fecundity, lifespan, reproduction time, and body size. |
| Zhou et al. [164] | Oryzias latipes | Nano-PS 100 nm | 3 months | Liver health impaired. |
| Felix et al. [166] | Danio Rerio | Micro-PP 200 μm | 21 days | Decreased mitochondrial health, anxiety-like behavior, and increased oxidative stress. |
| Trevisan et al. [167] | Danio Rerio | Nano-PS 44 nm | 24–48–96 h post-fertilization | Mitochondrial energy disruption, a decline in energy efficiency, and differential mitochondrial uptake. |
| Gu et al. [172] | Apostichopus japonicus | Nano-PS 100–200 nm | 20 days | Inhibits the complex activities in the mitochondrial respiratory chain and affect the relative expression levels of mitochondrial apoptosis-related genes. |
| Li et al. [186] | Chlamydomonas reinhardtii | Micro-PS | 10 days | Micro-PS had negative effects on growth and algal photosynthesis. |
| Besseling et al. [187] | Arenicola marina | Micro-PS 400–1300 μm | 28 days | As plastic is ingested by A. marina, its predators will be exposed to plastic as well. Weight loss. |
| Wang X et al. [188] | Mytilus coruscus | Micro-PS 2 μm | 14 days plus 7-day recovery acclimation | Inhibition of digestive enzymes. |
7. Ingestion of MPs/NPs from Aquatic Food by Human
8. Future Studies and Perspectives
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geremia, E.; Ripa, M.; Catone, C.M.; Ulgiati, S. A Review about Microalgae Wastewater Treatment for Bioremediation and Biomass Production—A New Challenge for Europe. Environments 2021, 8, 136. [Google Scholar] [CrossRef]
- Catone, C.M.; Ripa, M.; Geremia, E.; Ulgiati, S. Bio-Products from Algae-Based Biorefinery on Wastewater: A Review. J. Environ. Manag. 2021, 293, 112792. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Xu, Y.; Wang, Z. Review of OPFRs in Animals and Humans: Absorption, Bioaccumulation, Metabolism, and Internal Exposure Research. Chemosphere 2016, 153, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and Toxicity of Antibiotics in the Aquatic Environment: A Review. Chemosphere 2020, 251, 126351. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of Microplastics in Freshwater Systems: A Review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, G.A.; Gómez-Oliván, L.M. Microplastics in Aquatic Environments: A Review on Occurrence, Distribution, Toxic Effects, and Implications for Human Health. Sci. Total Environ. 2021, 780, 146551. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Sosa-Hernández, J.E.; Raza, A.; Nabeel, F.; Iqbal, H.M.N. Biosorption: An Interplay between Marine Algae and Potentially Toxic Elements—A Review. Mar. Drugs 2018, 16, 65. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Hemavathy, R.V.; Jeevanantham, S.; Harikumar, P.; Priyanka, G.; Devakirubai, D.R.A. A Comprehensive Review on Sources, Analysis and Toxicity of Environmental Pollutants and Its Removal Methods from Water Environment. Sci. Total Environ. 2022, 812, 152456. [Google Scholar] [CrossRef]
- Shahjahan, M.; Taslima, K.; Rahman, M.S.; Al-Emran, M.; Alam, S.I.; Faggio, C. Effects of Heavy Metals on Fish Physiology—A Review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef]
- Sfakianakis, D.G.; Renieri, E.; Kentouri, M.; Tsatsakis, A.M. Effect of Heavy Metals on Fish Larvae Deformities: A Review. Environ. Res. 2015, 137, 246–255. [Google Scholar] [CrossRef]
- Kahlon, S.K.; Sharma, G.; Julka, J.M.; Kumar, A.; Sharma, S.; Stadler, F.J. Impact of Heavy Metals and Nanoparticles on Aquatic Biota. Environ. Chem. Lett. 2018, 16, 919–946. [Google Scholar] [CrossRef]
- Turan, N.B.; Erkan, H.S.; Engin, G.O.; Bilgili, M.S. Nanoparticles in the Aquatic Environment: Usage, Properties, Transformation and Toxicity—A Review. Process Saf. Environ. Prot. 2019, 130, 238–249. [Google Scholar] [CrossRef]
- Nowack, B.; Bucheli, T.D. Occurrence, Behavior and Effects of Nanoparticles in the Environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Lovern, S.B.; Klaper, R. Daphnia Magna Mortality When Exposed to Titanium Dioxide and Fullerene (C60) Nanoparticles. Environ. Toxicol. Chem. 2006, 25, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy Metals in Marine Fish Meat and Consumer Health: A Review. J. Sci. Food Agric. 2016, 96, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Capriello, T.; Venditti, P.; Fasciolo, G.; La Pietra, A.; Trifuoggi, M.; Giarra, A.; Agnisola, C.; Ferrandino, I. Aluminum Induces a Stress Response in Zebrafish Gills by Influencing Metabolic Parameters, Morphology, and Redox Homeostasis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 271, 109633. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Fasciolo, G.; Venditti, P. Mitochondrial Management of Reactive Oxygen Species. Antioxidants 2021, 10, 1824. [Google Scholar] [CrossRef]
- Javed, M.; Usmani, N. An Overview of the Adverse Effects of Heavy Metal Contamination on Fish Health. Proc. Natl. Acad. Sci. India Sect. B-Biol. Sci. 2019, 89, 389–403. [Google Scholar] [CrossRef]
- Afshan, S.; Ali, S.; Shaista Ameen, U.; Farid, M.; Aslam Bharwana, S.; Hannan, F.; Ahmad, R.; Pakistan, F. Effect of Different Heavy Metal Pollution on Fish. Res. J. Chem. Environ. Sci. 2014, 2, 74–79. [Google Scholar]
- Fogliano, C.; Motta, C.M.; Venditti, P.; Fasciolo, G.; Napolitano, G.; Avallone, B.; Carotenuto, R. Environmental Concentrations of a Delorazepam-Based Drug Impact on Embryonic Development of Non-Target Xenopus Laevis. Aquat. Toxicol. 2022, 250, 106244. [Google Scholar] [CrossRef]
- Gothwal, R.; Shashidhar, T. Antibiotic Pollution in the Environment: A Review. Clean 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Liu, J.L.; Wong, M.H. Pharmaceuticals and Personal Care Products (PPCPs): A Review on Environmental Contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, H.M.N. Environmentally-Related Contaminants of High Concern: Potential Sources and Analytical Modalities for Detection, Quantification, and Treatment. Environ. Int. 2019, 122, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global Trends in Antimicrobial Use in Food Animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Ying, G.G.; Su, H.C.; Stauber, J.L.; Adams, M.S.; Binet, M.T. Growth-Inhibiting Effects of 12 Antibacterial Agents and Their Mixtures on the Freshwater Microalga Pseudokirchneriella Subcapitata. Environ. Toxicol. Chem. 2008, 27, 1201–1208. [Google Scholar] [CrossRef]
- Binh, V.N.; Dang, N.; Anh, N.T.K.; Ky, L.X.; Thai, P.K. Antibiotics in the Aquatic Environment of Vietnam: Sources, Concentrations, Risk and Control Strategy. Chemosphere 2018, 197, 438–450. [Google Scholar] [CrossRef]
- Yi, X.; Lin, C.; Ong, E.J.L.; Wang, M.; Zhou, Z. Occurrence and Distribution of Trace Levels of Antibiotics in Surface Waters and Soils Driven by Non-Point Source Pollution and Anthropogenic Pressure. Chemosphere 2019, 216, 213–223. [Google Scholar] [CrossRef]
- Moore, M.N. Do Nanoparticles Present Ecotoxicological Risks for the Health of the Aquatic Environment? Environ. Int. 2006, 32, 967–976. [Google Scholar] [CrossRef]
- Umulisa, V.; Kalisa, D.; Skutlarek, D.; Reichert, B. First Evaluation of DDT (Dichlorodiphenyltrichloroethane) Residues and Other Persistence Organic Pollutants in Soils of Rwanda: Nyabarongo Urban versus Rural Wetlands. Ecotoxicol. Environ. Saf. 2020, 197, 110574. [Google Scholar] [CrossRef]
- Mansouri, A.; Cregut, M.; Abbes, C.; Durand, M.J.; Landoulsi, A.; Thouand, G. The Environmental Issues of DDT Pollution and Bioremediation: A Multidisciplinary Review. Appl. Biochem. Biotechnol. 2016, 181, 309–339. [Google Scholar] [CrossRef]
- Teklu, B.M.; Haileslassie, A.; Mekuria, W. Pesticides as Water Pollutants and Level of Risks to Environment and People: An Example from Central Rift Valley of Ethiopia. Environ. Dev. Sustain. 2022, 24, 5275–5294. [Google Scholar] [CrossRef]
- Islam, M.A.; Amin, S.M.N.; Rahman, M.A.; Juraimi, A.S.; Uddin, M.K.; Brown, C.L.; Arshad, A. Chronic Effects of Organic Pesticides on the Aquatic Environment and Human Health: A Review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100740. [Google Scholar] [CrossRef]
- Thuy, T.T. Effects of DDT on Environment and Human Health. J. Educ. Soc. Sci. 2015, 2, 108–114. [Google Scholar]
- Who Global Malaria Programme. The Use of DDT in Malaria Vector Control WHO Position Statement; World Health Organization: Basel, Switzerland, 2011. [Google Scholar]
- Owens, K.D.; Baer, K.N. Modifications of the Topical Japanese Medaka (Oryzias latipes) Embryo Larval Assay for Assessing Developmental Toxicity of Pentachlorophenol and p, P′-Dichlorodiphenyltrichloroethane. Ecotoxicol. Environ. Saf. 2000, 47, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ton, C.; Lin, Y.; Willett, C. Zebrafish as a Model for Developmental Neurotoxicity Testing. Birth Defects Res. A Clin. Mol. Teratol. 2006, 76, 553–567. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and Importance of Microplastics in the Marine Environment: A Review of the Sources, Fate, Effects, and Potential Solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef]
- Lu, H.C.; Ziajahromi, S.; Neale, P.A.; Leusch, F.D.L. A Systematic Review of Freshwater Microplastics in Water and Sediments: Recommendations for Harmonisation to Enhance Future Study Comparisons. Sci. Total Environ. 2021, 781, 146693. [Google Scholar] [CrossRef]
- VOSviewer—Visualizing Scientific Landscapes. Available online: https://www.vosviewer.com/ (accessed on 1 August 2023).
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in Fisheries and Aquaculture Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO: Rome, Italy, 2017. [Google Scholar]
- Zhao, S.; Zettler, E.R.; Bos, R.P.; Lin, P.; Amaral-Zettler, L.A.; Mincer, T.J. Large Quantities of Small Microplastics Permeate the Surface Ocean to Abyssal Depths in the South Atlantic Gyre. Glob. Chang. Biol. 2022, 28, 2991–3006. [Google Scholar] [CrossRef]
- Murano, C.; Vaccari, L.; Casotti, R.; Corsi, I.; Palumbo, A. Occurrence of Microfibres in Wild Specimens of Adult Sea Urchin Paracentrotus lividus (Lamarck, 1816) from a Coastal Area of the Central Mediterranean Sea. Mar. Pollut. Bull. 2022, 176, 113448. [Google Scholar] [CrossRef]
- Murano, C.; Nonnis, S.; Scalvini, F.G.; Maffioli, E.; Corsi, I.; Tedeschi, G.; Palumbo, A. Response to Microplastic Exposure: An Exploration into the Sea Urchin Immune Cell Proteome. Environ. Pollut. 2023, 320, 121062. [Google Scholar] [CrossRef]
- Macias, D.; Cózar, A.; Garcia-Gorriz, E.; González-Fernández, D.; Stips, A. Surface Water Circulation Develops Seasonally Changing Patterns of Floating Litter Accumulation in the Mediterranean Sea. A Modelling Approach. Mar. Pollut. Bull. 2019, 149, 110619. [Google Scholar] [CrossRef]
- Jiang, B.; Kauffman, A.E.; Li, L.; McFee, W.; Cai, B.; Weinstein, J.; Lead, J.R.; Chatterjee, S.; Scott, G.I.; Xiao, S. Health Impacts of Environmental Contamination of Micro- and Nanoplastics: A Review. Environ. Health Prev. Med. 2020, 25, 29. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A Bacterium That Degrades and Assimilates Poly(Ethylene Terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Okoffo, E.D.; O’Brien, S.; O’Brien, J.W.; Tscharke, B.J.; Thomas, K.V. Wastewater Treatment Plants as a Source of Plastics in the Environment: A Review of Occurrence, Methods for Identification, Quantification and Fate. Environ. Sci. Water Res. Technol. 2019, 5, 1908–1931. [Google Scholar] [CrossRef]
- Jiang, J.Q. Occurrence of Microplastics and Its Pollution in the Environment: A Review. Sustain. Prod. Consum. 2018, 13, 16–23. [Google Scholar] [CrossRef]
- FAO. Microplastics in Fisheries and Aquaculture: What Do We Know? Should We Be Worried? FAO: Rome, Italy, 2019. [Google Scholar]
- Bergmann, M.; Gutow, L.; Klages, M. Marine Anthropogenic Litter; Springer Nature: Berlin/Heidelberg, Germany, 2015; pp. 1–447. [Google Scholar] [CrossRef]
- Murano, C.; Agnisola, C.; Caramiello, D.; Castellano, I.; Casotti, R.; Corsi, I.; Palumbo, A. How Sea Urchins Face Microplastics: Uptake, Tissue Distribution and Immune System Response. Environ. Pollut. 2020, 264, 114685. [Google Scholar] [CrossRef]
- Junaid, M.; Wang, J. Interaction of Nanoplastics with Extracellular Polymeric Substances (EPS) in the Aquatic Environment: A Special Reference to Eco-Corona Formation and Associated Impacts. Water Res. 2021, 201, 117319. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Wu, F.; Niu, L.; Tang, Z.; Liang, W.; Zhao, T.; Fang, M.; Wang, H.; Wang, X. Characterization, Occurrence, Environmental Behaviors, and Risks of Nanoplastics in the Aquatic Environment: Current Status and Future Perspectives. Fundam. Res. 2021, 1, 317–328. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Bottero, J.Y.; Lowry, G.V.; Jolivet, J.P.; Wiesner, M.R. Towards a Definition of Inorganic Nanoparticles from an Environmental, Health and Safety Perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, Q.; Chen, X.; Wang, R.; Duan, M.; Wu, C. Occurrence of Microplastic in the Water of Different Types of Aquaculture Ponds in an Important Lakeside Freshwater Aquaculture Area of China. Chemosphere 2021, 282, 131126. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Y.; Leung, J.Y.S.; Huang, W.; Zeng, J.; Tang, Y.; Chen, J.; Shi, A.; Yu, X.; Xu, X.; et al. Accumulation of Microplastics in Typical Commercial Aquatic Species: A Case Study at a Productive Aquaculture Site in China. Sci. Total Environ. 2020, 708, 135432. [Google Scholar] [CrossRef]
- Enrichetti, F.; Bavestrello, G.; Betti, F.; Rindi, F.; Tregrosso, A.; Bo, M. Fate of Lost Fishing Gears: Experimental Evidence of Biofouling Colonization Patterns from the Northwestern Mediterranean Sea. Environ. Pollut. 2021, 268, 115746. [Google Scholar] [CrossRef]
- Gajanur, A.R.; Jaafar, Z. Abandoned, Lost, or Discarded Fishing Gear at Urban Coastlines. Mar. Pollut. Bull. 2022, 175, 113341. [Google Scholar] [CrossRef]
- Gajanur, A.R.; Jaafar, Z. Behaviour of Stranded Abandoned Plastic Fishing Nets in the Equatorial Tropics. Nat. Singap. 2023, 16, e2023034. [Google Scholar] [CrossRef]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Ocean. 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Lehtiniemi, M.; Hartikainen, S.; Näkki, P.; Engström-Öst, J.; Koistinen, A.; Setälä, O. Size Matters More than Shape: Ingestion of Primary and Secondary Microplastics by Small Predators. Food Webs 2018, 17, e00097. [Google Scholar] [CrossRef]
- Murano, C.; Palumbo, A.; Leone, S. Toxicological Impacts of Microplastics: Effects on Levels of Cellular Thiols in Mytilus Galloprovincialis. Environ. Toxicol. Chem. 2023, 42, 1607–1613. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Mekonnen, B.A.; Aragaw, T.A.; Mekonnen, B.A.; Mekonnen Bahir, B.A. Distribution and Impact of Microplastics in the Aquatic Systems: A Review of Ecotoxicological Effects on Biota. In Microplastic Pollution. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry; Springer: Berlin/Heidelberg, Germany, 2021; pp. 65–104. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Ma, X.; Guo, B.; Zhang, K. Environmental Factors-Mediated Behavior of Microplastics and Nanoplastics in Water: A Review. Chemosphere 2021, 271, 129597. [Google Scholar] [CrossRef]
- Velzeboer, I.; Kwadijk, C.J.A.F.; Koelmans, A.A. Strong Sorption of PCBs to Nanoplastics, Microplastics, Carbon Nanotubes, and Fullerenes. Environ. Sci. Technol. 2014, 48, 4869–4876. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yang, Q.; Jiang, J.; Dalu, T.; Kadushkin, A.; Singh, J.; Fakhrullin, R.; Wang, F.; Cai, X.; Li, R. Coronas of Micro/Nano Plastics: A Key Determinant in Their Risk Assessments. Part. Fibre Toxicol. 2022, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Corsi, I. Effects of Nanomaterials on Marine Invertebrates. Sci. Total Environ. 2016, 565, 933–940. [Google Scholar] [CrossRef]
- Corsi, I.; Bergami, E.; Grassi, G. Behavior and Bio-Interactions of Anthropogenic Particles in Marine Environment for a More Realistic Ecological Risk Assessment. Front. Environ. Sci. 2020, 8, 524966. [Google Scholar] [CrossRef]
- Yu, F.; Yang, C.; Zhu, Z.; Bai, X.; Ma, J. Adsorption Behavior of Organic Pollutants and Metals on Micro/Nanoplastics in the Aquatic Environment. Sci. Total Environ. 2019, 694, 133643. [Google Scholar] [CrossRef] [PubMed]
- Romera-Castillo, C.; Letscher, R.T.; Hansell, D.A. New Nutrients Exert Fundamental Control on Dissolved Organic Carbon Accumulation in the Surface Atlantic Ocean. Proc. Natl. Acad. Sci. USA 2016, 113, 10497–10502. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Le, C.; Chiu, M.H.; Chin, W.C. The Impact of Nanoplastics on Marine Dissolved Organic Matter Assembly. Sci. Total Environ. 2018, 634, 316–320. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “Plastisphere”: Microbial Communities on Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Sooriyakumar, P.; Bolan, N.; Kumar, M.; Singh, L.; Yu, Y.; Li, Y.; Weralupitiya, C.; Vithanage, M.; Ramanayaka, S.; Sarkar, B.; et al. Biofilm Formation and Its Implications on the Properties and Fate of Microplastics in Aquatic Environments: A Review. J. Hazard. Mater. Adv. 2022, 6, 100077. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Labrenz, M. Marine Microbial Assemblages on Microplastics: Diversity, Adaptation, and Role in Degradation. Annu. Rev. Mar. Sci. 2020, 12, 209–232. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.A.; Zettler, E.R.; Mincer, T.J. Ecology of the Plastisphere. Nat. Rev. Microbiol. 2020, 18, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, I.V.; Kirmizi, S.; Wichels, A.; Garin-Fernandez, A.; Erler, R.; Löder, M.; Gerdts, G. Dangerous Hitchhikers? Evidence for Potentially Pathogenic Vibrio Spp. on Microplastic Particles. Mar. Environ. Res. 2016, 120, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vroom, R.J.E.; Koelmans, A.A.; Besseling, E.; Halsband, C. Aging of Microplastics Promotes Their Ingestion by Marine Zooplankton. Environ. Pollut. 2017, 231, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.; Smith, K.E.; Lewis, C. The Sea Urchin Paracentrotus Lividus as a Bioeroder of Plastic. Sci. Total Environ. 2019, 693, 133621. [Google Scholar] [CrossRef]
- Casotti, R.; Palumbo, A.; Murano, C.; Donnarumma, V.; Corsi, I. Impact of Microbial Colonization of Polystyrene Microbeads on the Toxicological Responses in the Sea Urchin Paracentrotus Lividus. Environ. Sci. Technol. 2021, 55, 7990–8000. [Google Scholar] [CrossRef]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of Microplastic Debris throughout the Marine Ecosystem. Nat. Ecol. Evol. 2017, 1, 116. [Google Scholar] [CrossRef]
- Bulleri, F.; Ravaglioli, C.; Anselmi, S.; Renzi, M. The Sea Cucumber Holothuria Tubulosa Does Not Reduce the Size of Microplastics but Enhances Their Resuspension in the Water Column. Sci. Total Environ. 2021, 781, 146650. [Google Scholar] [CrossRef]
- Choy, C.A.; Robison, B.H.; Gagne, T.O.; Erwin, B.; Firl, E.; Halden, R.U.; Hamilton, J.A.; Katija, K.; Lisin, S.E.; Rolsky, C.; et al. The Vertical Distribution and Biological Transport of Marine Microplastics across the Epipelagic and Mesopelagic Water Column. Sci. Rep. 2019, 9, 7843. [Google Scholar] [CrossRef]
- Kooi, M.; Van Nes, E.H.; Scheffer, M.; Koelmans, A.A. Ups and Downs in the Ocean: Effects of Biofouling on Vertical Transport of Microplastics. Environ. Sci. Technol. 2017, 51, 7963–7971. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic Debris in the Open Ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Hu, M.; Palić, D. Micro- and Nano-Plastics Activation of Oxidative and Inflammatory Adverse Outcome Pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Fasciolo, G.; Venditti, P. The Ambiguous Aspects of Oxygen. Oxygen 2022, 2, 382–409. [Google Scholar] [CrossRef]
- Rai, P.K.; Sonne, C.; Brown, R.J.C.; Younis, S.A.; Kim, K.H. Adsorption of Environmental Contaminants on Micro- and Nano-Scale Plastic Polymers and the Influence of Weathering Processes on Their Adsorptive Attributes. J. Hazard. Mater. 2022, 427, 127903. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Li, Y.; Gao, J.; Cao, R.; Shang, E.; Zhang, W. ROS-Mediated Photoaging Pathways of Nano- and Micro-Plastic Particles under UV Irradiation. Water Res. 2022, 216, 118320. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Jia, H.; Sun, Y.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Long-Term Phototransformation of Microplastics under Simulated Sunlight Irradiation in Aquatic Environments: Roles of Reactive Oxygen Species. Water Res. 2020, 173, 115564. [Google Scholar] [CrossRef]
- Urli, S.; Corte Pause, F.; Crociati, M.; Baufeld, A.; Monaci, M.; Stradaioli, G. Impact of Microplastics and Nanoplastics on Livestock Health: An Emerging Risk for Reproductive Efficiency. Animals 2023, 13, 1132. [Google Scholar] [CrossRef]
- Riesbeck, S. Do Microplastics Induce Oxidative Stress in Marine Invertebrates? Available online: https://epic.awi.de/id/eprint/48395/ (accessed on 21 October 2022).
- Wu, H.; Xu, T.; Chen, T.; Liu, J.; Xu, S. Oxidative Stress Mediated by the TLR4/NOX2 Signalling Axis Is Involved in Polystyrene Microplastic-Induced Uterine Fibrosis in Mice. Sci. Total Environ. 2022, 838, 155825. [Google Scholar] [CrossRef]
- Fasciolo, G.; Napolitano, G.; Aprile, M.; Cataldi, S.; Costa, V.; Tomajoli, M.T.M.; Lombardi, A.; Di Meo, S.; Venditti, P. Muscle Oxidative Stress Plays a Role in Hyperthyroidism-Linked Insulin Resistance. Antioxidants 2023, 12, 592. [Google Scholar] [CrossRef]
- Fasciolo, G.; Napolitano, G.; Aprile, M.; Cataldi, S.; Costa, V.; Ciccodicola, A.; Di Meo, S.; Venditti, P. Hepatic Insulin Resistance in Hyperthyroid Rat Liver: Vitamin E Supplementation Highlights a Possible Role of ROS. Antioxidants 2022, 11, 1295. [Google Scholar] [CrossRef]
- Jahnke, A.; Arp, H.P.H.; Escher, B.I.; Gewert, B.; Gorokhova, E.; Kühnel, D.; Ogonowski, M.; Potthoff, A.; Rummel, C.; Schmitt-Jansen, M.; et al. Reducing Uncertainty and Confronting Ignorance about the Possible Impacts of Weathering Plastic in the Marine Environment. Environ. Sci. Technol. Lett. 2017, 4, 85–90. [Google Scholar] [CrossRef]
- Celina, M.C. Review of Polymer Oxidation and Its Relationship with Materials Performance and Lifetime Prediction. Polym. Degrad. Stab. 2013, 98, 2419–2429. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and Photostabilization of Polymers, Especially Polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed]
- Pannetier, P.; Cachot, J.; Clérandeau, C.; Faure, F.; Van Arkel, K.; de Alencastro, L.F.; Levasseur, C.; Sciacca, F.; Bourgeois, J.P.; Morin, B. Toxicity Assessment of Pollutants Sorbed on Environmental Sample Microplastics Collected on Beaches: Part I-Adverse Effects on Fish Cell Line. Environ. Pollut. 2019, 248, 1088–1097. [Google Scholar] [CrossRef]
- Lee, S.E.; Yi, Y.; Moon, S.; Yoon, H.; Park, Y.S. Impact of Micro- and Nanoplastics on Mitochondria. Metabolites 2022, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Balk, J.; Leaver, C.J.; McCabe, P.F. Translocation of Cytochrome c from the Mitochondria to the Cytosol Occurs during Heat-Induced Programmed Cell Death in Cucumber Plants. FEBS Lett. 1999, 463, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Li, T.; Chen, K.; Deng, X.; Zhang, Q.; Tang, H.; Shi, Z.; Zhu, T.; Zhu, J. PS-NPs Induced Neurotoxic Effects in SHSY-5Y Cells via Autophagy Activation and Mitochondrial Dysfunction. Brain Sci. 2022, 12, 952. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Li, M.; Jiang, Q.; Wu, D.; Huang, Y.; Jiao, Y.; Zhang, M.; Zhao, Y. Effects of Nanoplastics on Antioxidant and Immune Enzyme Activities and Related Gene Expression in Juvenile Macrobrachium nipponense. J. Hazard. Mater. 2020, 398, 122990. [Google Scholar] [CrossRef]
- Ferrante, M.C.; Monnolo, A.; Del Piano, F.; Raso, G.M.; Meli, R. The Pressing Issue of Micro- and Nanoplastic Contamination: Profiling the Reproductive Alterations Mediated by Oxidative Stress. Antioxidants 2022, 11, 193. [Google Scholar] [CrossRef]
- Arihilam, N.H.; Arihilam, E.C. Impact and Control of Anthropogenic Pollution on the Ecosystem—A Review. J. Biosci. Biotechnol. Discov. 2019, 4, 2536–7064. [Google Scholar] [CrossRef]
- Bukola, D.; Zaid, A.; Olalekan, E.I.; Falilu, A. Consequences of Anthropogenic Activities on Fish and the Aquatic Environment. Poult. Fish. Wildl. Sci. 2015, 3, 2. [Google Scholar] [CrossRef]
- Gray, J.S. Marine Biodiversity: Patterns, Threats and Conservation Needs. Available online: https://www.sciencebase.gov/catalog/item/50539dc6e4b097cd4fce79f5 (accessed on 4 July 2023).
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Prakash, S. Impact of Climate Change on Aquatic Ecosystem and Its Biodiversity: An Overview. Available online: http://ijbi.org.in/papers/10.%20IJBI%20Dec%202021%20Dr%20Sadguru.pdf (accessed on 2 July 2023).
- Nava, V.; Leoni, B. A Critical Review of Interactions between Microplastics, Microalgae and Aquatic Ecosystem Function. Water Res. 2021, 188, 116476. [Google Scholar] [CrossRef] [PubMed]
- Karapanagioti, H.H. Hazardous Chemicals Associated with Plastics in the Marine Environment; Springer International Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in Sediments: A Review of Techniques, Occurrence and Effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Moriceau, B.; Gallinari, M.; Lambert, C.; Huvet, A.; Raffray, J.; Soudant, P. Interactions between Microplastics and Phytoplankton Aggregates: Impact on Their Respective Fates. Mar. Chem. 2015, 175, 39–46. [Google Scholar] [CrossRef]
- Wang, Z.; Su, B.; Xu, X.; Di, D.; Huang, H.; Mei, K.; Dahlgren, R.A.; Zhang, M.; Shang, X. Preferential Accumulation of Small (<300 μm) Microplastics in the Sediments of a Coastal Plain River Network in Eastern China. Water Res. 2018, 144, 393–401. [Google Scholar] [CrossRef]
- Wang, S.; Liu, M.; Wang, J.; Huang, J.; Wang, J. Polystyrene Nanoplastics Cause Growth Inhibition, Morphological Damage and Physiological Disturbance in the Marine Microalga Platymonas helgolandica. Mar. Pollut. Bull. 2020, 158, 111403. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Effects of Microplastics on Microalgae Populations: A Critical Review. Sci. Total Environ. 2019, 665, 400–405. [Google Scholar] [CrossRef]
- Khatiwada, J.R.; Madsen, C.; Warwick, C.; Shrestha, S.; Chio, C.; Qin, W. Interaction between Polyethylene Terephthalate (PET) Microplastic and Microalgae (Scenedesmus spp.): Effect on the Growth, Chlorophyll Content, and Hetero-Aggregation. Environ. Adv. 2023, 13, 100399. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Wang, J.; Tan, L. Toxic Effects of Microplastic on Marine Microalgae Skeletonema Costatum: Interactions between Microplastic and Algae. Environ. Pollut. 2017, 220, 1282–1288. [Google Scholar] [CrossRef]
- Guschina, I.A.; Hayes, A.J.; Ormerod, S.J. Polystyrene Microplastics Decrease Accumulation of Essential Fatty Acids in Common Freshwater Algae. Environ. Pollut. 2020, 263, 114425. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Li, Y.; Li, J.; Wang, F.; Xia, S.; Zhao, J. Biofilm Alters Tetracycline and Copper Adsorption Behaviors onto Polyethylene Microplastics. Chem. Eng. J. 2020, 392, 123808. [Google Scholar] [CrossRef]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental Occurrences, Fate, and Impacts of Microplastics. Ecotoxicol. Environ. Saf. 2019, 184, 109612. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Liddle, C.; Consolandi, G.; Drago, C.; Hird, C.; Lindeque, P.K.; Galloway, T.S. Microplastics, Microfibres and Nanoplastics Cause Variable Sub-Lethal Responses in Mussels (Mytilus spp.). Mar. Pollut. Bull. 2020, 160, 111552. [Google Scholar] [CrossRef] [PubMed]
- Wegner, A.; Besseling, E.; Foekema, E.M.; Kamermans, P.; Koelmans, A.A. Effects of Nanopolystyrene on the Feeding Behavior of the Blue Mussel (Mytilus Edulis L.). Environ. Toxicol. Chem. 2012, 31, 2490–2497. [Google Scholar] [CrossRef] [PubMed]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster Reproduction Is Affected by Exposure to Polystyrene Microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef]
- Murray, F.; Cowie, P.R. Plastic Contamination in the Decapod Crustacean Nephrops Norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 2011, 62, 1207–1217. [Google Scholar] [CrossRef]
- Helfman, G.S.; Collette, B.B.; Facey, D.E.; Bowen, B.W. The Diversity of Fishes: Biology, Evolution, and Ecology; Helfman, G.S., Collette, B.B., Facey, D.E., Bowen, B.W., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; Available online: https://books.google.it/books?hl=it&lr=&id=FyehAR6hsUUC&oi=fnd&pg=PR7&dq=The+diversity+of+fishes:+Biology,+evolution,+and+ecology.+&ots=lqBp8z5KZy&sig=Cf4pwwIp65J_-_BkdqMIQ6Ir48A&redir_esc=y#v=onepage&q=The%20diversity%20of%20fishes%3A%20Biology%2C%20evolution%2C%20and%20ecology.&f=false (accessed on 4 July 2023).
- Holmlund, C.M.; Hammer, M. Ecosystem Services Generated by Fish Populations. Ecol. Econ. 1999, 29, 253–268. [Google Scholar] [CrossRef]
- Lynch, A.J.; Myers, B.J.E.; Chu, C.; Eby, L.A.; Falke, J.A.; Kovach, R.P.; Krabbenhoft, T.J.; Kwak, T.J.; Lyons, J.; Paukert, C.P.; et al. Climate Change Effects on North American Inland Fish Populations and Assemblages. Fisheries 2016, 41, 346–361. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic Ingestion by Zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Kumar, A.; Neale, P.A.; Leusch, F.D.L. Impact of Microplastic Beads and Fibers on Waterflea (Ceriodaphnia dubia) Survival, Growth, and Reproduction: Implications of Single and Mixture Exposures. Environ. Sci. Technol. 2017, 51, 13397–13406. [Google Scholar] [CrossRef]
- Rehse, S.; Kloas, W.; Zarfl, C. Short-Term Exposure with High Concentrations of Pristine Microplastic Particles Leads to Immobilisation of Daphnia magna. Chemosphere 2016, 153, 91–99. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The Impact of Polystyrene Microplastics on Feeding, Function and Fecundity in the Marine Copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef]
- Cole, M.; Galloway, T.S. Ingestion of Nanoplastics and Microplastics by Pacific Oyster Larvae. Environ. Sci. Technol. 2015, 49, 14625–14632. [Google Scholar] [CrossRef]
- Zheng, S.; Zhao, Y.; Liangwei, W.; Liang, J.; Liu, T.; Zhu, M.; Li, Q.; Sun, X. Characteristics of Microplastics Ingested by Zooplankton from the Bohai Sea, China. Sci. Total Environ. 2020, 713, 136357. [Google Scholar] [CrossRef]
- Karami, A.; Romano, N.; Galloway, T.; Hamzah, H. Virgin Microplastics Cause Toxicity and Modulate the Impacts of Phenanthrene on Biomarker Responses in African Catfish (Clarias gariepinus). Environ. Res. 2016, 151, 58–70. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, G.; Song, W.; Ye, C.; Lin, H.; Li, Z.; Liu, W. Plastics in the Marine Environment Are Reservoirs for Antibiotic and Metal Resistance Genes. Environ. Int. 2019, 123, 79–86. [Google Scholar] [CrossRef]
- Gigault, J.; Ter Halle, A.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Ephanie Reynaud, S. Current Opinion: What Is a Nanoplastic? *. Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Bucci, K.; Tulio, M.; Rochman, C.M. What Is Known and Unknown about the Effects of Plastic Pollution: A Meta-Analysis and Systematic Review. Ecol. Appl. 2020, 30, e02044. [Google Scholar] [CrossRef]
- Kögel, T.; Bjorøy, Ø.; Toto, B.; Bienfait, A.M.; Sanden, M. Micro- and Nanoplastic Toxicity on Aquatic Life: Determining Factors. Sci. Total Environ. 2020, 709, 136050. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Rillig, M.C. Effects of Microplastic Fibers and Drought on Plant Communities. Environ. Sci. Technol. 2020, 54, 6166–6173. [Google Scholar] [CrossRef]
- Felten, V.; Toumi, H.; Masfaraud, J.F.; Billoir, E.; Camara, B.I.; Férard, J.F. Microplastics Enhance Daphnia Magna Sensitivity to the Pyrethroid Insecticide Deltamethrin: Effects on Life History Traits. Sci. Total Environ. 2020, 714, 136567. [Google Scholar] [CrossRef]
- Agathokleous, E.; Iavicoli, I.; Barceló, D.; Calabrese, E.J. Micro/Nanoplastics Effects on Organisms: A Review Focusing on ‘Dose’. J. Hazard. Mater. 2021, 417, 126084. [Google Scholar] [CrossRef]
- Routti, H.; Harju, M.; Lühmann, K.; Aars, J.; Ask, A.; Goksøyr, A.; Kovacs, K.M.; Lydersen, C. Concentrations and Endocrine Disruptive Potential of Phthalates in Marine Mammals from the Norwegian Arctic. Environ. Int. 2021, 152, 106458. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.S.; Lee, Y.K.; Po, B.H.K.; Wong, L.C.; Xu, X.; Wong, C.F.; Wong, C.Y.; Tam, N.F.Y.; Cheung, S.G. Impacts of Typhoon Mangkhut in 2018 on the Deposition of Marine Debris and Microplastics on Beaches in Hong Kong. Sci. Total Environ. 2020, 716, 137172. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of Microplastics by Commercial Fish off the Portuguese Coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef]
- Wardrop, P.; Shimeta, J.; Nugegoda, D.; Morrison, P.D.; Miranda, A.; Tang, M.; Clarke, B.O. Chemical Pollutants Sorbed to Ingested Microbeads from Personal Care Products Accumulate in Fish. Environ. Sci. Technol. 2016, 50, 4037–4044. [Google Scholar] [CrossRef]
- Redondo-Hasselerharm, P.E.; Falahudin, D.; Peeters, E.T.H.M.; Koelmans, A.A. Microplastic Effect Thresholds for Freshwater Benthic Macroinvertebrates. Environ. Sci. Technol. 2018, 52, 2278–2286. [Google Scholar] [CrossRef]
- Straub, S.; Hirsch, P.E.; Burkhardt-Holm, P. Biodegradable and Petroleum-Based Microplastics Do Not Differ in Their Ingestion and Excretion but in Their Biological Effects in a Freshwater Invertebrate Gammarus Fossarum. Int. J. Environ. Res. Public Health 2017, 14, 774. [Google Scholar] [CrossRef]
- König Kardgar, A.; Ghosh, D.; Sturve, J.; Agarwal, S.; Carney Almroth, B. Chronic Poly(l-Lactide) (PLA)- Microplastic Ingestion Affects Social Behavior of Juvenile European Perch (Perca fluviatilis). Sci. Total Environ. 2023, 881, 163425. [Google Scholar] [CrossRef]
- Moore, C.J.; Lattin, G.L.; Zellers, A.F. Working Our Way Upstream: A Snapshot of Land-Based Contributions of Plastic and Other Trash to Coastal Waters and Beaches of Southern California. In Proceedings of the Plastic Debris Rivers to Sea Conference, Algalita Marine Research Foundation, Long Beach, CA, USA, 8 September 2005. [Google Scholar]
- Draper, A.M.; Weissburg, M.J. Impacts of Global Warming and Elevated CO2 on Sensory Behavior in Predator-Prey Interactions: A Review and Synthesis. Front. Ecol. Evol. 2019, 7, 428445. [Google Scholar] [CrossRef]
- Wang, J.; Tan, Z.; Peng, J.; Qiu, Q.; Li, M. The Behaviors of Microplastics in the Marine Environment. Mar. Environ. Res. 2016, 113, 7–17. [Google Scholar] [CrossRef]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of Microplastics in the Gastrointestinal Tract of Pelagic and Demersal Fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Romeo, T.; Pietro, B.; Pedà, C.; Consoli, P.; Andaloro, F.; Fossi, M.C. First Evidence of Presence of Plastic Debris in Stomach of Large Pelagic Fish in the Mediterranean Sea. Mar. Pollut. Bull. 2015, 95, 358–361. [Google Scholar] [CrossRef] [PubMed]
- United Nations Tuna Day|United Nations. Available online: https://www.un.org/en/observances/tuna-day (accessed on 6 July 2023).
- Diaz-Basantes, M.F.; Nacimba-Aguirre, D.; Conesa, J.A.; Fullana, A. Presence of Microplastics in Commercial Canned Tuna. Food Chem. 2022, 385, 132721. [Google Scholar] [CrossRef] [PubMed]
- Di Giacinto, F.; Di Renzo, L.; Mascilongo, G.; Notarstefano, V.; Gioacchini, G.; Giorgini, E.; Bogdanović, T.; Petričević, S.; Listeš, E.; Brkljača, M.; et al. Detection of Microplastics, Polymers and Additives in Edible Muscle of Swordfish (Xiphias gladius) and Bluefin Tuna (Thunnus thynnus) Caught in the Mediterranean Sea. J. Sea Res. 2023, 192, 102359. [Google Scholar] [CrossRef]
- Abihssira-García, I.S.; Park, Y.; Kiron, V.; Olsvik, P.A. Fluorescent Microplastic Uptake by Immune Cells of Atlantic Salmon (Salmo salar L.). Front. Environ. Sci. 2020, 8, 560206. [Google Scholar] [CrossRef]
- Batel, A.; Linti, F.; Scherer, M.; Erdinger, L.; Braunbeck, T. Transfer of Benzo[a]Pyrene from Microplastics to Artemia Nauplii and Further to Zebrafish via a Trophic Food Web Experiment: CYP1A Induction and Visual Tracking of Persistent Organic Pollutants. Environ. Toxicol. Chem. 2016, 35, 1656–1666. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Response to Comment on “Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver”. Environ. Sci. Technol. 2016, 50, 12523–12524. [Google Scholar] [CrossRef]
- Jeong, C.B.; Won, E.J.; Kang, H.M.; Lee, M.C.; Hwang, D.S.; Hwang, U.K.; Zhou, B.; Souissi, S.; Lee, S.J.; Lee, J.S. Microplastic Size-Dependent Toxicity, Oxidative Stress Induction, and p-JNK and p-P38 Activation in the Monogonont Rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef]
- Manabe, M.; Tatarazako, N.; Kinoshita, M. Uptake, Excretion and Toxicity of Nano-Sized Latex Particles on Medaka (Oryzias latipes) Embryos and Larvae. Aquat. Toxicol. 2011, 105, 576–581. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, L.; Xu, H.; Xu, E.G.; Li, M.; Wang, Y. Long-Term Exposure to Polystyrene Nanoplastics Impairs the Liver Health of Medaka. Water 2022, 14, 2767. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested Plastic Transfers Hazardous Chemicals to Fish and Induces Hepatic Stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef] [PubMed]
- Félix, L.; Carreira, P.; Peixoto, F. Effects of Chronic Exposure of Naturally Weathered Microplastics on Oxidative Stress Level, Behaviour, and Mitochondrial Function of Adult Zebrafish (Danio rerio). Chemosphere 2023, 310, 136895. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, R.; Voy, C.; Chen, S.; Di Giulio, R.T. Nanoplastics Decrease the Toxicity of a Complex PAH Mixture but Impair Mitochondrial Energy Production in Developing Zebrafish. Environ. Sci. Technol. 2019, 53, 8405–8415. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.N.; Leung, M.C.K.; Rooney, J.P.; Sendoel, A.; Hengartner, M.O.; Kisby, G.E.; Bess, A.S. Mitochondria as a Target of Environmental Toxicants. Toxicol. Sci. 2013, 134, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Raftery, T.D.; Jayasundara, N.; Di Giulio, R.T. A Bioenergetics Assay for Studying the Effects of Environmental Stressors on Mitochondrial Function in Vivo in Zebrafish Larvae. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 192, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, C.D.; Jayasundara, N.; Kozal, J.S.; Leuthner, T.C.; Di Giulio, R.T. Resistance to Polycyclic Aromatic Hydrocarbon Toxicity and Associated Bioenergetic Consequences in a Population of Fundulus Heteroclitus. Ecotoxicology 2017, 26, 435–448. [Google Scholar] [CrossRef]
- Massarsky, A.; Jayasundara, N.; Bailey, J.M.; Oliveri, A.N.; Levin, E.D.; Prasad, G.L.; Di Giulio, R.T. Teratogenic, Bioenergetic, and Behavioral Effects of Exposure to Total Particulate Matter on Early Development of Zebrafish (Danio rerio) Are Not Mimicked by Nicotine. Neurotoxicol. Teratol. 2015, 51, 77–88. [Google Scholar] [CrossRef]
- Gu, Y.; Xu, D.; Liu, J.; Chen, Y.; Wang, J.; Song, Y.; Sun, B.; Xia, B. Bioaccumulation of Functionalized Polystyrene Nanoplastics in Sea Cucumber Apostichopus Japonicus (Selenka, 1867) and Their Toxic Effects on Oxidative Stress, Energy Metabolism and Mitochondrial Pathway. Environ. Pollut. 2023, 319, 121015. [Google Scholar] [CrossRef]
- Guimarães, A.T.B.; Charlie-Silva, I.; Malafaia, G. Toxic Effects of Naturally-Aged Microplastics on Zebrafish Juveniles: A More Realistic Approach to Plastic Pollution in Freshwater Ecosystems. J. Hazard. Mater. 2021, 407, 124833. [Google Scholar] [CrossRef]
- Bhagat, J.; Zang, L.; Kaneco, S.; Nishimura, N.; Shimada, Y. Combined Exposure to Nanoplastics and Metal Oxide Nanoparticles Inhibits Efflux Pumps and Causes Oxidative Stress in Zebrafish Embryos. Sci. Total Environ. 2022, 835, 155436. [Google Scholar] [CrossRef]
- Sendra, M.; Pereiro, P.; Yeste, M.P.; Mercado, L.; Figueras, A.; Novoa, B. Size Matters: Zebrafish (Danio rerio) as a Model to Study Toxicity of Nanoplastics from Cells to the Whole Organism. Environ. Pollut. 2021, 268, 115769. [Google Scholar] [CrossRef] [PubMed]
- Sarasamma, S.; Audira, G.; Siregar, P.; Malhotra, N.; Lai, Y.H.; Liang, S.T.; Chen, J.R.; Chen, K.H.C.; Hsiao, C. Der Nanoplastics Cause Neurobehavioral Impairments, Reproductive and Oxidative Damages, and Biomarker Responses in Zebrafish: Throwing up Alarms of Wide Spread Health Risk of Exposure. Int. J. Mol. Sci. 2020, 21, 1410. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.A.; Trevisan, R.; Massarsky, A.; Kozal, J.S.; Levin, E.D.; Di Giulio, R.T. Maternal Transfer of Nanoplastics to Offspring in Zebrafish (Danio rerio): A Case Study with Nanopolystyrene. Sci. Total Environ. 2018, 643, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarzadeh, F.; Rafiee, M.; Khodagholi, F.; Khorramizadeh, M.R.; Manouchehri, H.; Eslami, A.; Sayehmiri, F.; Mohseni-Bandpei, A. Adverse Effects of Polystyrene Nanoplastic and Its Binary Mixtures with Nonylphenol on Zebrafish Nervous System: From Oxidative Stress to Impaired Neurotransmitter System. Environ. Pollut. 2023, 317, 120587. [Google Scholar] [CrossRef]
- Umamaheswari, S.; Priyadarshinee, S.; Kadirvelu, K.; Ramesh, M. Polystyrene Microplastics Induce Apoptosis via ROS-Mediated P53 Signaling Pathway in Zebrafish. Chem. Biol. Interact. 2021, 345, 109550. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Santos, D.; Félix, L.; Luzio, A.; Parra, S.; Cabecinha, E.; Bellas, J.; Monteiro, S.M. Toxicological Effects Induced on Early Life Stages of Zebrafish (Danio rerio) after an Acute Exposure to Microplastics Alone or Co-Exposed with Copper. Chemosphere 2020, 261, 127748. [Google Scholar] [CrossRef]
- Yang, H.; Lai, H.; Huang, J.; Sun, L.; Mennigen, J.A.; Wang, Q.; Liu, Y.; Jin, Y.; Tu, W. Polystyrene Microplastics Decrease F–53B Bioaccumulation but Induce Inflammatory Stress in Larval Zebrafish. Chemosphere 2020, 255, 127040. [Google Scholar] [CrossRef]
- Chen, X.; Peng, L.B.; Wang, D.; Zhu, Q.L.; Zheng, J.L. Combined Effects of Polystyrene Microplastics and Cadmium on Oxidative Stress, Apoptosis, and GH/IGF Axis in Zebrafish Early Life Stages. Sci. Total Environ. 2022, 813, 152514. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics Induce Intestinal Inflammation, Oxidative Stress, and Disorders of Metabolome and Microbiome in Zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yuan, S.; Zhou, Y.; Li, X.; Duan, L.; Huang, L.; Zhou, X.; Ma, Y.; Pang, S. Microplastics Reduce the Bioaccumulation and Oxidative Stress Damage of Triazole Fungicides in Fish. Sci. Total Environ. 2022, 806, 151475. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, P.; Zhang, C.; Zhou, X.; Yin, Z.; Hu, T.; Hu, D.; Liu, C.; Zhu, L. Influence of Polystyrene Microplastics on the Growth, Photosynthetic Efficiency and Aggregation of Freshwater Microalgae Chlamydomonas Reinhardtii. Sci. Total Environ. 2020, 714, 136767. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Wegner, A.; Foekema, E.M.; Van Den Heuvel-Greve, M.J.; Koelmans, A.A. Effects of Microplastic on Fitness and PCB Bioaccumulation by the Lugworm Arenicola Marina (L.). Environ. Sci. Technol. 2013, 47, 593–600. [Google Scholar] [CrossRef]
- Wang, X.; Huang, W.; Wei, S.; Shang, Y.; Gu, H.; Wu, F.; Lan, Z.; Hu, M.; Shi, H.; Wang, Y. Microplastics Impair Digestive Performance but Show Little Effects on Antioxidant Activity in Mussels under Low PH Conditions. Environ. Pollut. 2020, 258, 113691. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in Bivalves Cultured for Human Consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Devriese, L.I.; van der Meulen, M.D.; Maes, T.; Bekaert, K.; Paul-Pont, I.; Frère, L.; Robbens, J.; Vethaak, A.D. Microplastic Contamination in Brown Shrimp (Crangon Crangon, Linnaeus 1758) from Coastal Waters of the Southern North Sea and Channel Area. Mar. Pollut. Bull. 2015, 98, 179–187. [Google Scholar] [CrossRef]
- Al-Thawadi, S. Microplastics and Nanoplastics in Aquatic Environments: Challenges and Threats to Aquatic Organisms. Arab. J. Sci. Eng. 2020, 45, 4419–4440. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The Plastic Brain: Neurotoxicity of Micro- and Nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef]
- Greven, A.C.; Merk, T.; Karagöz, F.; Mohr, K.; Klapper, M.; Jovanović, B.; Palić, D. Polycarbonate and Polystyrene Nanoplastic Particles Act as Stressors to the Innate Immune System of Fathead Minnow (Pimephales promelas). Environ. Toxicol. Chem. 2016, 35, 3093–3100. [Google Scholar] [CrossRef]
- Mattsson, K.; Ekvall, M.T.; Hansson, L.A.; Linse, S.; Malmendal, A.; Cedervall, T. Altered Behavior, Physiology, and Metabolism in Fish Exposed to Polystyrene Nanoparticles. Environ. Sci. Technol. 2015, 49, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yin, D.; Jia, Y.; Schiwy, S.; Legradi, J.; Yang, S.; Hollert, H. Enhanced Uptake of BPA in the Presence of Nanoplastics Can Lead to Neurotoxic Effects in Adult Zebrafish. Sci. Total Environ. 2017, 609, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wu, X.; Liu, S.; Wang, Z.; Chen, L. Size-Dependent Effects of Polystyrene Microplastics on Cytotoxicity and Efflux Pump Inhibition in Human Caco-2 cells. Chemosphere 2019, 221, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Bhuyan, M.S. Effects of Microplastics on Fish and in Human Health. Front. Environ. Sci. 2022, 10, 827289. [Google Scholar] [CrossRef]
- Dong, C.D.; Chen, C.W.; Chen, Y.C.; Chen, H.H.; Lee, J.S.; Lin, C.H. Polystyrene Microplastic Particles: In Vitro Pulmonary Toxicity Assessment. J. Hazard. Mater. 2020, 385, 121575. [Google Scholar] [CrossRef]
- Wang, F.; Bexiga, M.G.; Anguissola, S.; Boya, P.; Simpson, J.C.; Salvati, A.; Dawson, K.A. Time Resolved Study of Cell Death Mechanisms Induced by Amine-Modified Polystyrene Nanoparticles. Nanoscale 2013, 5, 10868–10876. [Google Scholar] [CrossRef]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and Toxicity: A Preliminary Study of Effects of Nanoplastic Particles on Human Lung Epithelial Cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef]
- Wick, P.; Malek, A.; Manser, P.; Meili, D.; Maeder-Althaus, X.; Diener, L.; Diener, P.A.; Zisch, A.; Krug, H.F.; Von Mandach, U. Barrier Capacity of Human Placenta for Nanosized Materials. Environ. Health Perspect. 2010, 118, 432–436. [Google Scholar] [CrossRef]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of Nanoplastic in the Environment and Possible Impact on Human Health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Tamayo-Belda, M.; Pulido-Reyes, G.; Amariei, G.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Secondary Nanoplastics Released from a Biodegradable Microplastic Severely Impact Freshwater Environments. Environ. Sci. Nano 2019, 6, 1382–1392. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, C. Biodegradable Plastics: Green Hope or Greenwashing? Mar. Pollut. Bull. 2020, 161, 111774. [Google Scholar] [CrossRef]
- Arantzamendi, L.; Andrés, M.; Basurko, O.C.; Suárez, M.J. Circular and Lower Impact Mussel and Seaweed Aquaculture by a Shift towards Bio-Based Ropes. Rev. Aquac. 2023, 15, 1010–1019. [Google Scholar] [CrossRef]
- Sandhya, M.; Aravind, J.; Kanmani, P. Production of Polyhydroxyalkanoates from Ralstonia Eutropha Using Paddy Straw as Cheap Substrate. Int. J. Environ. Sci. Technol. 2013, 10, 47–54. [Google Scholar] [CrossRef]
- Narayan Bharti, S.S.G. Need for Bioplastics and Role of Biopolymer PHB: A Short Review. Artic. J. Pet. Environ. Biotechnol. 2016, 7, 2. [Google Scholar] [CrossRef]
- Maheswari, N.U.; Ahilandeswari, K. Production of Bioplastic Using Spirulina Platensis and Comparison with Commercial Plastic. Res. Environ. Life Sci. 2011, 4, 133–136. [Google Scholar]
- Urtuvia, V.; Villegas, P.; González, M.; Seeger, M. Bacterial Production of the Biodegradable Plastics Polyhydroxyalkanoates. Int. J. Biol. Macromol. 2014, 70, 208–213. [Google Scholar] [CrossRef]
- Napolitano, G.; Venditti, P.; Agnisola, C.; Quartucci, S.; Fasciolo, G.; Muscari Tomajoli, M.T.; Geremia, E.; Catone, C.M.; Ulgiati, S. Towards Sustainable Aquaculture Systems: Biological and Environmental Impact of Replacing Fishmeal with Arthrospira Platensis (Nordstedt) (Spirulina). J. Clean Prod. 2022, 374, 133978. [Google Scholar] [CrossRef]
- Harding, K.G.; Dennis, J.S.; von Blottnitz, H.; Harrison, S.T.L. Environmental Analysis of Plastic Production Processes: Comparing Petroleum-Based Polypropylene and Polyethylene with Biologically-Based Poly-β-Hydroxybutyric Acid Using Life Cycle Analysis. J. Biotechnol. 2007, 130, 57–66. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geremia, E.; Muscari Tomajoli, M.T.; Murano, C.; Petito, A.; Fasciolo, G. The Impact of Micro- and Nanoplastics on Aquatic Organisms: Mechanisms of Oxidative Stress and Implications for Human Health—A Review. Environments 2023, 10, 161. https://doi.org/10.3390/environments10090161
Geremia E, Muscari Tomajoli MT, Murano C, Petito A, Fasciolo G. The Impact of Micro- and Nanoplastics on Aquatic Organisms: Mechanisms of Oxidative Stress and Implications for Human Health—A Review. Environments. 2023; 10(9):161. https://doi.org/10.3390/environments10090161
Chicago/Turabian StyleGeremia, Eugenio, Maria Teresa Muscari Tomajoli, Carola Murano, Adriana Petito, and Gianluca Fasciolo. 2023. "The Impact of Micro- and Nanoplastics on Aquatic Organisms: Mechanisms of Oxidative Stress and Implications for Human Health—A Review" Environments 10, no. 9: 161. https://doi.org/10.3390/environments10090161
APA StyleGeremia, E., Muscari Tomajoli, M. T., Murano, C., Petito, A., & Fasciolo, G. (2023). The Impact of Micro- and Nanoplastics on Aquatic Organisms: Mechanisms of Oxidative Stress and Implications for Human Health—A Review. Environments, 10(9), 161. https://doi.org/10.3390/environments10090161