Elevated Blood Lead Levels in Children Associated with Living near Mining Waste Sites in Guerrero/Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Communities Selection
2.2. Community Behavioral Analysis
2.3. BBL Determination from Human Samples
2.4. Collection of Soil Samples and Lead Determination
2.5. Data Analysis
3. Results
3.1. Pb Levels in Soil are High in Communities near Mining Waste Tailings
3.2. BLL Comparison in Near and Far Communities from Waste Tailings
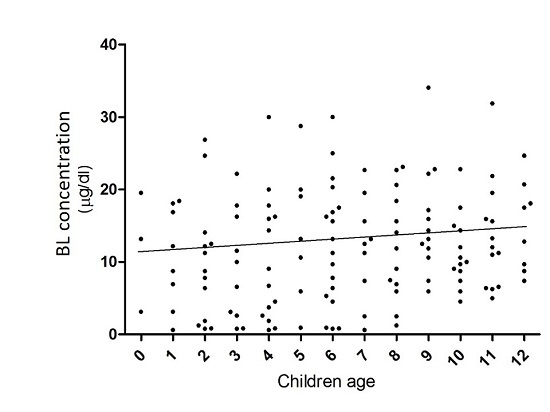
3.3. Age Influence in Lead Concentrations
3.4. Occupational and Other Variations that Affect Lead Concentration
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kianoush, S.; Sadeghi, M.; Balali-Mood, M. Recent advances in the clinical management of lead poisoning. Acta Med. Iran. 2015, 53, 327–336. [Google Scholar] [PubMed]
- Beamer, P.; Key, M.E.; Ferguson, A.C.; Canales, R.A.; Auyeung, W.; Leckie, J.O. Quantified activity pattern data from 6 to 27-month-old farmworker children for use in exposure assessment. Environ. Res. 2008, 108, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Mehrparvar, A.; Aghilinejad, M. Appendectomy due to lead poisoning: A case-report. J. Occup. Med. Toxicol. 2008, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Vega-Franco, L.; Alvear, G.; Meza-Camacho, C. Glazed pottery as a risk factor in lead exposure. Salud Publica Mex. 1994, 36, 148–153. [Google Scholar] [PubMed]
- Azcona-Cruz, M.I.; Rothenberg, S.J.; Schnaas-Arrieta, L.; Romero-Placeres, M.; Perroni-Hernandez, E. Levels of plasmatic lead in children 8–10 years of age and its relation to changes in visual-motor system and balance. Salud Publica Mex. 2000, 42, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, R.A. Occupational lead poisoning: 2-Chemical signs of the absorption of lead. J. Occup. Med. 1972, 14, 390–396. [Google Scholar] [PubMed]
- Bjerre, B.; Berglund, M.; Harsbo, K.; Hellman, B. Blood lead concentrations of swedish preschool children in a community with high lead levels from mine waste in soil and dust. Scand. J. Work Environ. Health 1993, 19, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Danse, I.H.; Garb, L.G.; Moore, R.H. Blood lead surveys of communities in proximity to lead-containing mill tailings. Am. Ind. Hyg. Assoc. J. 1995, 56, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Rieuwerts, J.S.; Farago, M.E.; Cikrt, M.; Bencko, V. Differences in lead bioavailability between a smelting and a mining area. Water Air Soil Pollut. 2000, 122, 203–229. [Google Scholar] [CrossRef]
- Steele, M.J.; Beck, B.D.; Murphy, B.L.; Strauss, H.S. Assessing the contribution from lead in mining wastes to blood lead. Regul. Toxicol. Pharmacol. 1990, 11, 158–190. [Google Scholar] [CrossRef]
- Murgueytio, A.M.; Evans, R.G.; Roberts, D. Relationship between soil and dust lead in a lead mining area and blood lead levels. J. Expo. Anal. Environ. Epidemiol. 1998, 8, 173–186. [Google Scholar] [PubMed]
- Gulson, B.L.; Mizon, K.J.; Korsch, M.J.; Howarth, D. Importance of monitoring family members in establishing sources and pathways of lead in blood. Sci. Total Environ. 1996, 188, 173–182. [Google Scholar] [CrossRef]
- Malcoe, L.H.; Lynch, R.A.; Keger, M.C.; Skaggs, V.J. Lead sources, behaviors, and socioeconomic factors in relation to blood lead of native American and white children: A community-based assessment of a former mining area. Environ. Health Perspect. 2002, 110, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Savely, S.M.; Emery, R.J.; Connor, T.H. A comparison of methods for determining lead content in drinking water: A portable anodic stripping voltammetry instrument method versus the standard epa 239.2 method. AIHAJ 2000, 61, 557–562. [Google Scholar] [CrossRef]
- Sterling, D.A.; Lewis, R.D.; Luke, D.A.; Shadel, B.N. A portable x-ray fluorescence instrument for analyzing dust wipe samples for lead: Evaluation with field samples. Environ. Res. 2000, 83, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Talavera-Mendoza, O. Determination of Bioavailable Metals in Mining Shafts as a Guide to Assess their Potential for Contamination; Memorias del III; Foro de Estudios Sobre Guerrero: Acapulco, Mexico, 2000. [Google Scholar]
- PaceScan 3000 TM Operator. Operator Manual Instructions for Using the Pacescan System for Quantitative Analysis of Lead in Paint, Dust, Soil And Drinking Water; Pace Environs, Inc.: Lehigh Valley, PA, USA, 1997. [Google Scholar]
- Soto-R#xED;os, M.J.C.; Talavera, O.; Aguilar, G.; Rothenberg, S. Living near mining waste is associated with higher blood lead concentrations in children. In Proceedings of the Memories International Worshop, Buenos Aires, Argentina, 29–30 August 2005. [Google Scholar]
- Meza-Figueroa, D.; Maier, R.M.; de la, O.V.M.; Gomez-Alvarez, A.; Moreno-Zazueta, A.; Rivera, J.; Campillo, A.; Grandlic, C.J.; Anaya, R.; Palafox-Reyes, J. The impact of unconfined mine tailings in residential areas from a mining town in a semi-arid environment: Nacozari, Sonora, Mexico. Chemosphere 2009, 77, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, C.; Romieu, I.; Palazuelos, E.; Munoz, I.; Cortes, M.; Rivero, A.; Catalan, J. Environmental exposure factors and the concentrations of blood lead in Mexico city children. Salud Publica Mex. 1993, 35, 599–606. [Google Scholar] [PubMed]
- Romieu, I.; Carreon, T.; Lopez, L.; Palazuelos, E.; Rios, C.; Manuel, Y.; Hernandez-Avila, M. Environmental urban lead exposure and blood lead levels in children of Mexico city. Environ. Health Perspect. 1995, 103, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Schnaas, L.; Rothenberg, S.J.; Flores, M.F.; Martinez, S.; Hernandez, C.; Osorio, E.; Perroni, E. Blood lead secular trend in a cohort of children in Mexico city (1987–2002). Environ. Health Perspect. 2004, 112, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Mattuck, R.L.; Beck, B.D.; Bowers, T.S.; Cohen, J.T. Recent trends in childhood blood lead levels. Arch. Environ. Health 2001, 56, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Medlin, J. Sweet candy, bitter poison. Environ. Health Perspect. 2004, 112, A803. [Google Scholar] [CrossRef] [PubMed]
- Jasso-Pineda, Y.; Diaz-Barriga, F.; Calderon, J.; Yanez, L.; Carrizales, L.; Perez-Maldonado, I.N. DNA damage and decreased DNA repair in peripheral blood mononuclear cells in individuals exposed to arsenic and lead in a mining site. Biol. Trace Elem. Res. 2012, 146, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Paoliello, M.M.; De Capitani, E.M. Occupational and environmental human lead exposure in Brazil. Environ. Res 2007, 103, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Jalili, M.; Azizkhani, R. Lead toxicity resulting from chronic ingestion of opium. West. J. Emergency Med. 2009, 10, 244–246. [Google Scholar]
- Mexicana, N.O. Nom-157-semarnat-2009. Diario Oficial de la Federación. 2011. Available online: http://www.dof.gob.mx/normasOficiales/4485/semarnat1/semarnat1.htm (accessed on 2 June 2017).


| Environmental Samples | Exposed | Non-Exposed | ||
|---|---|---|---|---|
| GM (range) | GM (range) | |||
| A | B | C | D | |
| Distance from mining waste sites (km) | 0.1 < 5 | 0.2 < 5 | 5–10 | >35 |
| Soil (ppm) | 32.2(1, 1480) | 6.9 (1, 284) | 2.1 (1, 47) | 2.1 (1, 47) |
| n = 7 | n = 14 | n = 5 | n = 5 | |
| Water (μg/L) | 2.0 (2, 2) | 1.8 (1, 2) | 2.0 ( 2, 2) | 2.0 (2, 2) |
| n = 6 | n = 7 | n = 4 | n = 7 | |
| Exposed | Non Exposed | |||
|---|---|---|---|---|
| Groups * | GM (95% CI) | GM (95% CI) | ||
| A | B | C | D | |
| n = 27 | n = 59 | n = 29 | n = 41 | |
| Total group a | 13.6(10.6, 17.4) | 15.9 (14.7,17.2) | 5.5 (3.9, 7.7) | 5.5 (4.1, 7.4) |
| Girls b | 14.9 (11.5, 19.3) | 15.6 (14.1, 17.3) | 5.1 (3.3, 7.6) | 5.5 (3.3, 9.1) |
| Boys b | 11.2 (6.1, 20.8) | 16.2 (14.2, 18.4) | 5.3 (2.7, 10.2) | 4.6 (2.6, 8.1) |
| <6 years of Age b | 10.8 (7.2, 16.4) | 16.1 (14.2, 18.1) | 5.2 (2.1, 12.8) | 3.7 (2.1, 6.7) |
| ≥6 years of Age b | 18.0 (15.5, 20.9) | 15.8 (14.2, 17.6) | 5.6 (3.9, 8.2) | 7.1 (5.3, 9.7) |
| Use of glazed ceramics | ||||
| Yes a | 15.8 (12.8, 19.6) | 16.0 (14.5, 17.6) | 5.2 (3.2, 8.5) | 8.1 (6.7, 9.8) |
| No a | 10.2 (5.5, 18.9) | 15.7 (13.2, 18.6) | 4.8 (1.7, 13.2) | 2.0 (0.8, 5.0) |
| Attends school | ||||
| Yes b | 14.2 (9.7, 20.8) | 15.9 (14.4, 17.5) | 6.0 (3.9, 9.2) | 8.1(6.41,10.2) |
| No b | 12.6 (8.5, 18.9) | 15.9 (14.1, 17.9) | 2.8 (0.7, 11.2) | 2.0 (0.7, 5.3) |
| Home workshop | ||||
| Yes a | 18.3 (14.1, 23.8) | 17.3 (15.0, 20.0) | - | - |
| No a | 12.4 (9.1, 17.0) | 15.2 (13.8, 16.8) | 5.1 (3.3, 7.7) | 4.9 (3.3, 7.3) |
| Variables | Coefficients | 95% CI | p > t |
|---|---|---|---|
| Cooking in glazed ceramics b | 1.9 | −0.3, 4.1 | 0.193 |
| Sex (feminine) b | −0.9 | −2.8, 1.1 | 0.362 |
| Age a | 0.4 | 0.1, 0.7 | 0.009 |
| Community A b | 8.2 | 5.2, 11.1 | 0 |
| Community B b | 8.1 | 5.5, 10.6 | 0 |
| Community C b | −0.3 | −2.9, 2.4 | 0.848 |
| Constant | 4.8 | 0.9, 8.7 | 0.016 |
| Community | OR | 95% CI |
|---|---|---|
| Community A | 7.3 | 2.1, 25.1 a |
| Community B | 80.5 | 9.7, 665.9 a |
| Community C | 0.7 | 0.2, 2.1 |
| Cooking in glazed ceramics | 1.7 | 0.6, 4.8 |
| Sex (feminine) | 1 | 0.4, 2.6 |
| Age | 1.1 | 0.9, 1.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Ríos, M.D.L.; Juárez-Pérez, C.A.; Rendón-Gandarilla, F.J.; Talavera-Mendoza, O.; Aguilar-Madrid, G. Elevated Blood Lead Levels in Children Associated with Living near Mining Waste Sites in Guerrero/Mexico. Environments 2017, 4, 41. https://doi.org/10.3390/environments4020041
Soto-Ríos MDL, Juárez-Pérez CA, Rendón-Gandarilla FJ, Talavera-Mendoza O, Aguilar-Madrid G. Elevated Blood Lead Levels in Children Associated with Living near Mining Waste Sites in Guerrero/Mexico. Environments. 2017; 4(2):41. https://doi.org/10.3390/environments4020041
Chicago/Turabian StyleSoto-Ríos, María De Lourdes, Cuauhtémoc Arturo Juárez-Pérez, Francisco Javier Rendón-Gandarilla, Oscar Talavera-Mendoza, and Guadalupe Aguilar-Madrid. 2017. "Elevated Blood Lead Levels in Children Associated with Living near Mining Waste Sites in Guerrero/Mexico" Environments 4, no. 2: 41. https://doi.org/10.3390/environments4020041
APA StyleSoto-Ríos, M. D. L., Juárez-Pérez, C. A., Rendón-Gandarilla, F. J., Talavera-Mendoza, O., & Aguilar-Madrid, G. (2017). Elevated Blood Lead Levels in Children Associated with Living near Mining Waste Sites in Guerrero/Mexico. Environments, 4(2), 41. https://doi.org/10.3390/environments4020041