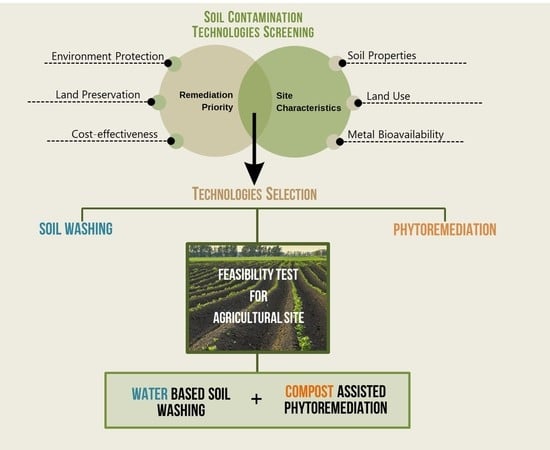
Comparative Evaluation of Technologies at a Heavy Metal Contaminated Site: The Role of Feasibility Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Site
2.2. Soil Analysis
2.3. Soil Washing Feasibility Test
- (1)
- To make a slurry, water was added to the contaminated soil sample in a liquid-to-solid ratio of 3:1 (defined after preliminary experiments) in 2 L plastic vessels.
- (2)
- To detach the finer particles from the coarser ones, the slurry was shaken at 20 °C overnight in a high-speed agitator to enable the vigorous brushing of the soil particles.
- (3)
- The slurry was then screened using different sieves to separate the soil particles by size.
- (4)
- At the end of the test, the contaminated soil was separated into the following fractions: >5 mm, 5–2 mm, 2–0.2 mm, 0.2–0.1 mm, 0.1–0.063 mm, <0.063 mm.
- (5)
- Each fraction was recovered and weighed after oven drying at 105 °C, and Zn, Cd, and Ni were determined in each fraction from the test and in the washing water using inductively coupled plasma optical emission spectroscopy (ICP-OES) with a Liberty AX Varian spectrometer.
2.4. Phytoextraction Feasibility Test
- Mesocosms with 3 kg of original, untreated soil (U-soil);
- Mesocosms with 3 kg of 10% compost-treated soil (T-soil);
- The mesocosms were sown with 1.5 g of Brassica juncea seeds or 10 seeds of Zea mays per pot;
- Daily irrigation according to the needs of the plants;
- Pots are equipped with a leachate collector to evaluate the possible leaching of the metals.
2.5. Metal Analysis
2.6. Quality Assurance and Quality Control
2.7. Statistical Analysis
3. Results and Discussion
3.1. Results from Soil Washing Test
3.2. Results of Phytoextraction Feasibility Test
3.3. Selection of Technologies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pietrelli, L.; Ferro, S.; Reverberi, A.P.; Vocciante, M. Removal and recovery of heavy metals from tannery sludge subjected to plasma pyro-gasification process. J. Clean. Prod. 2020, 273, 123166. [Google Scholar] [CrossRef]
- Cirrincione, L.; Di Dio, S.; Peri, G.; Scaccianoce, G.; Schillaci, D.; Rizzo, G. A win-win scheme for improving the environmental sustainability of university commuters’ mobility and getting environmental credits. Energies 2022, 15, 396. [Google Scholar] [CrossRef]
- Vocciante, M.; Meshalkin, V. An accurate inverse model for the detection of leaks in sealed landfills. Sustainability 2020, 12, 5598. [Google Scholar] [CrossRef]
- Cirrincione, L.; La Gennusa, M.; Peri, G.; Rizzo, G.; Scaccianoce, G. The landfilling of municipal solid waste and the sustainability of the related transportation activities. Sustainability 2022, 14, 5272. [Google Scholar] [CrossRef]
- Cachada, A.; Rocha-Santos, T.A.P.; Duarte, A.C. Soil and pollution: An introduction to the main issues. In Soil Pollution: From Monitoring to Remediation; Duarte, A.C., Cachada, A., Rocha-Santos, T.A.P., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–28. [Google Scholar]
- Farhadian, M.; Vachelard, C.; Duchez, D.; Larroche, C. In situ bioremediation of monoaromatic pollutants in groundwater: A review. Bioresour. Technol. 2008, 99, 5296–5308. [Google Scholar] [CrossRef] [PubMed]
- Pietrelli, L.; Ferro, S.; Reverberi, A.P.; Vocciante, M. Removal of polyethylene glycols from wastewater: A comparison of different approaches. Chemosphere 2021, 273, 129725. [Google Scholar] [CrossRef]
- Vocciante, M.; De Folly D’Auris, A.; Reverberi, A.P. A novel graphene-based sorbent for oil spill cleanup. Materials 2022, 5, 609. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Vocciante, M.; Salerno, M.; Soda, O.; Fabiano, B. A sustainable, top-down mechanosynthesis of carbohydrate-functionalized silver nanoparticles. React. Chem. Eng. 2022, 7, 888–897. [Google Scholar] [CrossRef]
- Khan, F.I.; Husain, T.; Hejazi, R. An overview and analysis of site remediation technologies. J. Environ. Manag. 2004, 71, 95–122. [Google Scholar] [CrossRef]
- Pavel, L.V.; Gavrilescu, M. Overview of ex situ decontamination techniques for soil cleanup. Environ. Eng. Manag. J. 2008, 7, 815–834. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent advances in soil remediation technology for heavy metal contaminated sites: A critical review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Sauer, T.J.; Cruse, R.M. Soil: The Forgotten Piece of the Water, Food, Energy Nexus; Sparks Advances in Agronomy; Donald, L., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 143, pp. 1–46. [Google Scholar] [CrossRef]
- Grifoni, M.; Franchi, E.; Fusini, D.; Vocciante, M.; Barbafieri, M.; Pedron, F.; Rosellini, I.; Petruzzelli, G. Soil Remediation: Towards a Resilient and Adaptive Approach to Deal with the Ever-Changing Environmental Challenges. Environments 2022, 9, 18. [Google Scholar] [CrossRef]
- Zerizghi, T.; Guo, Q.; Tian, L.; Wei, R.; Zhao, C. An integrated approach to quantify ecological and human health risks of soil heavy metal contamination around coal mining area. Sci. Total Environ. 2022, 814, 152653. [Google Scholar] [CrossRef] [PubMed]
- Kadriu, S.; Sadiku, M.; Kelmendi, M.; Sadriu, E. Studying the heavy metals concentration in discharged water from the Trepça Mine and flotation, Kosovo. Min. Miner. Depos. 2020, 14, 47–52. [Google Scholar] [CrossRef]
- Sadiku, M.; Kadriu, S.; Kelmendi, M.; Latifi, L. Impact of Artana mine on heavy metal pollution of the Marec river in Kosovo. Min. Miner. Depos. 2021, 15, 18–24. [Google Scholar] [CrossRef]
- Sadovenko, I.; Ulytsky, O.; Zahrytsenko, A.; Boiko, K. Risk assessment of radionuclide contamination spreading while flooding coal mined-out rocks. Min. Miner. Depos. 2020, 14, 130–136. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Ronghua, L.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- O’Connor, D.; Zheng, X.; Hou, D.; Shen, Z.; Li, G.; Miao, G.; O’Connell, S.; Guo, M. Phytoremediation: Climate change resilience and sustainability assessment at a coastal brownfield redevelopment. Environ. Int. 2019, 130, 104945. [Google Scholar] [CrossRef]
- Vocciante, M.; Reverberi, A.P.; Dovì, V.G. Approximate solution of the inverse Richards’ problem. Appl. Math. Model. 2016, 40, 5364–5376. [Google Scholar] [CrossRef]
- Petruzzelli, G.; Gorini, F.; Pezzarossa, B.; Pedron, F. The fate of pollutants in soil. In CNR Environment and Health Inter-Departmental Project: Present Knowledge and Prospects for Future Research; CNR: Rome, Italy, 2010; pp. 1–38. ISBN 978-88-8080-113-9. [Google Scholar]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512, 143–153. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Peng, M.; Cheng, X. Contamination Levels and the Ecological and Human Health Risks of Potentially Toxic Elements (PTEs) in Soil of Baoshan Area, Southwest China. Appl. Sci. 2022, 12, 1693. [Google Scholar] [CrossRef]
- İpek, M.; Ünlü, K. Development of human health risk-based Soil Quality Standards for Turkey: Conceptual framework. Environ. Adv. 2020, 1, 100004. [Google Scholar] [CrossRef]
- Hou, D.; O’Connor, D.; Igalavithana, A.D.; Alessi, D.S.; Luo, J.; Tsang, D.C.W.; Sparks, D.L.; Yamauchi, Y.; Rinklebe, J.; Ok, Y.S. Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat. Rev. Earth Environ. 2020, 1, 366–381. [Google Scholar] [CrossRef]
- Italian Ministerial Decree D.M. n. 46 of 1 March 2019; Official Gazette: Rome, Italy, 2019.
- García-Rubio, A.; Rodríguez-Maroto, J.M.; Gómez-Lahoz, C.; García-Herruzo, F.; Vereda-Alonso, C. Electrokinetic remediation: The use of mercury speciation for feasibility studies applied to a contaminated soil from Almadén. Electrochim. Acta 2011, 56, 9303–9310. [Google Scholar] [CrossRef]
- Chakrabartty, M.; Harun-Or-Rashid, G.M. Feasibility Study of the Soil Remediation Technologies in the Natural Environment. Am. J. Civ. Eng. 2021, 9, 91–98. [Google Scholar] [CrossRef]
- Rahim, F.; Abdullah, S.R.S.; Hasan, H.A.; Kurniawan, S.B.; Mamat, A.; Yusof, K.A.; Ambak, K.I. A feasibility study for the treatment of 1,2-dichloroethane-contaminated groundwater using reedbed system and assessment of its natural attenuation. Sci. Total Environ. 2022, 814, 152799. [Google Scholar] [CrossRef]
- Kaur, H.; Garg, N. Zinc toxicity in plants: A review. Planta 2021, 253, 129. [Google Scholar] [CrossRef]
- Balafrej, H.; Bogusz, D.; Triqui, Z.-E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc Hyperaccumulation in Plants: A Review. Plants 2020, 9, 562. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; whether toxic or essential for plants and environment—A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Ashraf, M. Essential roles and hazardous effects of nickel in plants. Rev. Environ. Contam. Toxicol. 2011, 214, 125–167. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.V.; Passarelli, M.N.; Newcomb, P.A. Cadmium exposure and cancer mortality in the Third National Health and Nutrition Examination Survey cohort. Occup. Environ. Med. 2012, 69, 153–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, A.; Patra, A.; Jatav, H.S.; Jatav, S.S.; Singh, S.K.; Sathyanarayana, E.; Verma, S.; Singh, P. Toxicity of Cadmium in Soil-Plant-Human Continuum and Its Bioremediation Techniques. In Soil Contamination—Threats and Sustainable Solutions; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis; Part 3. Chemical Methods; Soil Science Society of America Book Series; Sparks, D.L., Ed.; Soil Science Society of America Inc.: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis; Part 3. Chemical Methods; Soil Science Society of America Book Series; Sparks, D.L., Ed.; Soil Science Society of America Inc.: Madison, WI, USA, 1996; pp. 1201–1230. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis, 2nd ed.; Part 1. Physical and Mineralogical Methods; Agronomy Monograph No. 9; Klute, A., Ed.; American Society of Agronomy/Soil Science Society of America: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis; Part 3. Chemical Methods; Soil Science Society of America Book Series; Sparks, D.L., Ed.; Soil Science Society of America Inc.: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Petruzzelli, G.; Barbafieri, M.; Bonomo, L.; Saponaro, S.; Milani, A.; Pedron, F. Bench Scale Evaluation of Soil Washing for Heavy Metal Contaminated Soil at a Former Manufactured Gas Plant Site. Bull. Environ. Contam. Toxicol. 2004, 73, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Pedron, F.; Petruzzelli, G.; Barbafieri, M.; Tassi, E. Strategies to use phytoextraction in very acidic soil contaminated by heavy metals. Chemosphere 2009, 75, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, I.; Grifoni, M.; Franchi, E.; Cardaci, A.; Pedron, F.; Barbafieri, M.; Petruzzelli, G.; Vocciante, M. Enhanced Lead Phytoextraction by Endophytes from Indigenous Plants. Soil Syst. 2021, 5, 55. [Google Scholar] [CrossRef]
- Grifoni, M.; Petruzzelli, G.; Barbafieri, M.; Rosellini, I.; Pedron, F. Soil quality protection at heavy metal-contaminated manufactured gas plant sites: Role of biological remediation. In Enhancing Cleanup of Environmental Pollutants; Naser, A.A., Gill, S.S., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 231–260. [Google Scholar]
- ISO 18763; 2016 Soil Quality—Determination of the Toxic Effects of Pollutants on Germination and Early Growth of Higher Plants. International Organization for Standardization: Geneva, Switzerland, 2016.
- EPA—U.S. Environmental Protection Agency. Method 3051A, Microwave assisted acid digestion of sediments, sludges, soils and oils. In Test Methods for Evaluating Solid Waste, 3rd ed.; U.S. Environmental Protection Agency: Washington, DC, USA, 1995. [Google Scholar]
- Da Silva, Y.J.; do Nascimento, C.W.; Biondi, C.M. Comparison of USEPA digestion methods to heavy metals in soil samples. Environ Monit Assess. 2014, 186, 47–53. [Google Scholar] [CrossRef]
- Haynes, W. Tukey’s Test. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Derakhshan Nejad, Z.; Jung, M.C.; Kim, K.H. Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology. Environ. Geochem. Health 2018, 40, 927–953. [Google Scholar] [CrossRef]
- Acar, Y.B.; Gale, R.J.; Alshawabkeh, A.N.; Marks, R.E.; Puppala, S.; Bricka, M.; Parker, R. Electrokinetic remediation: Basics and technology status. J. Hazard. Mater. 1995, 40, 117–137. [Google Scholar] [CrossRef]
- Reddy, K.R.; Danda, S.; Saichek, R.E. Complicating factors of using ethylenediamine tetraacetic acid to enhance electrokinetic remediation of multiple heavy metals in clayey soils. J. Environ. Eng. 2004, 130, 1357–1366. [Google Scholar] [CrossRef]
- Vocciante, M.; Dovì, V.G.; Ferro, S. Sustainability in ElectroKinetic Remediation Processes: A Critical Analysis. Sustainability 2021, 13, 770. [Google Scholar] [CrossRef]
- Dermont, G.; Bergeron, M.; Mercier, G.; Richer-Laflèche, M. Soil washing for metal removal: A review of physical/chemical technologies and field applications. J. Hazard. Mater. 2008, 152, 1–31. [Google Scholar] [CrossRef]
- FRTR—Federal Remediation Technologies Roundtable. Remediation Technologies Screening Matrix and Reference Guides, 4th ed.; FRTR: Washington, DC, USA, 2007. Available online: https://frtr.gov/matrix2/top_page.html (accessed on 15 September 2022).
- CLAIRE—Contaminated land: Applications in real environments. In Understanding Soil Washing; Technical Bulletin, TB13; Claire London: London, UK, 2007.
- Mariusz, G.; Dorota, K.; Barbara, K. New-Generation Washing Agents in Remediation of Metal-Polluted Soils and Methods for Washing Effluent Treatment: A Review. Int. J. Env. Res. Public Health 2020, 17, 6220. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, J.; Liu, X.; Yuan, L.; Liu, X.; Deng, H. Comparative study on washing effects of different washing agents and conditions on heavy metal contaminated soil. Surf. Interfaces 2021, 27, 101563. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Kanyerere, T.; Wang, Y.S.; Sun, M. Washing Reagents for Remediating Heavy-Metal-Contaminated Soil: A Review. Front. Earth Sci. 2022, 10, 901570. [Google Scholar] [CrossRef]
- Parmar, S.; Singh, V. Phytoremediation approaches for heavy metal pollution: A review. J. Plant Sci. Res. 2015, 2, 135. [Google Scholar]
- Pilon-Smits, E. Phytoremediation. Ann. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef]
- Pedron, F.; Petruzzelli, G. Green remediation strategies to improve the quality of contaminated soils. Chem. Ecol. 2011, 27, 89–95. [Google Scholar] [CrossRef]
- Grifoni, M.; Pedron, F.; Barbafieri, M.; Rosellini, I.; Petruzzelli, G.; Franchi, E. Sustainable valorization of biomass: From assisted phytoremediation to green energy production. In Handbook on Assisted and Amendments Enhanced Sustainable Remediation Technology; Vara Prasad, M.N., Ed.; John Wiley & Sons: Chichester, UK, 2021. [Google Scholar]
- Lombi, E.; Zhao, F.J.; Dunham, S.J.; McGrath, S.P. Phytoremediation of heavy metal contaminated soils: Natural hyperaccumulation versus chemically-enhanced phytoextraction. J. Environ. Qual. 2001, 30, 1919–1926. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Barbafieri, M.; Morelli, E.; Tassi, E.; Pedron, F.; Remorini, D.; Petruzzelli, G. Overcoming limitation of “recalcitrant areas” to phytoextraction process: The synergistic effects of exogenous cytokinins and nitrogen treatments. Sci. Total Environ. 2018, 639, 1520–1529. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, L.; Liu, Q.; Li, J.; Qiao, Z.; Sun, P.; Yang, Y. A critical review on soil washing during soil remediation for heavy metals and or-ganic pollutants. Int. J. Environ. Sci. Technol. 2022, 19, 601–624. [Google Scholar] [CrossRef]
- Tsang, D.C.; Hartley, N.R. Metal distribution and spectroscopic analysis after soil washing with chelating agents and humic substances. Environ. Sci. Pollut. Res. 2014, 21, 3987–3995. [Google Scholar] [CrossRef]
- Ko, I.; Chang, Y.Y.; Lee, C.H.; Kim, K.W. Assessment of pilot-scale acid washing of soil contaminated with As, Zn and Ni using the BCR three-step sequential extraction. J. Hazard. Mater. 2005, 127, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.-M.; Oh, C.-T.; Kim, G.-J.; Lee, C.-H.; Sung, K.-J. Changes in the physicochemical properties of soil according to soil remediation methods. J. Soil Groundw. Environ. 2012, 17, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, H.; Wang, H.; Li, Q.; Li, Y. Effect of soil washing on heavy metal removal and soil quality: A two-sided coin. Ecotoxicol. Environ. Saf. 2020, 203, 11098. [Google Scholar] [CrossRef]
- Im, J.; Yang, K.; Jho, E.H.; Nam, K. Effect of different soil washing solutions on bioavaila-bility of residual arsenic in soils and soil properties. Chemosphere 2015, 138, 253–258. [Google Scholar] [CrossRef]
- UN—United Nations. Transforming our world: The 2030 agenda for sustainable development. In A/RES/70/1 Resolution Adopted by the General Assembly on 25 September 2015; UN General Assembly: New York, NY, USA, 2015; pp. 1–41. [Google Scholar]
- EC—European Commission. The European Green Deal—COM (2019) 640 Final; European Commission: Brussels, Belgium, 2019; pp. 1–24. [Google Scholar]
- EC—European Commission. Pathway to a Healthy Planet for All EU Action Plan: “Towards Zero Pollution for Air, Water and Soil”—COM (2021) 400 Final; European Commission: Brussels, Belgium, 2021; pp. 1–22. [Google Scholar]
- Adesodun, J.K.; Atayese, M.O.; Agbaje, T.; Osadiaye, B.A.; Mafe, O.; Soretire, A.A. Phytoremediation potentials of sunflowers (Tithonia Diversifolia and Helianthus Annuus) for metals in soils contaminated with zinc and lead ni-trates. Water Air Soil Pollut. 2010, 207, 195–201. [Google Scholar] [CrossRef]
- Marchiol, L.; Fellet, G.; Perosa, D.; Zerbi, G. Removal of trace metals by Sorghum Bicolor and Helianthus Annuus in a site polluted by industrial wastes: A field experience. Plant Physiol. Biochem. 2007, 45, 379–387. [Google Scholar] [CrossRef]
- Ximenez-Embun, P.; Rodriguez-Sanz, B.; Madrid-Albarran, Y.; C’amara, C. Uptake of heavy metals by lupin plants in artificially contaminated sand: Preliminary results. Int. J. Environ. Anal. Chem. 2002, 82, 805–814. [Google Scholar] [CrossRef]
- Liu, D.; Jiang, W.; Liu, C.; Xin, C.; Hou, W. Uptake and accumulation of lead by roots, hypocotyls and shoots of Indian mustard [Brassica juncea (L.)]. Bioresour. Technol. 2000, 71, 273–277. [Google Scholar] [CrossRef]
- Aleixandre-Benavent, R.; Aleixandre-Tudo, J.; Castello-Cogollos, L.; Aleixandre, J. Trends in scientific re-search on climate change in agriculture and forestry subject areas (2005–2014). J. Clean. Prod. 2017, 147, 406–418. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, H.; Szemmelveisz, K. Disposal options for polluted plants grown on heavy metal contaminated brownfield lands a review. Chemosphere 2017, 166, 8–20. [Google Scholar] [CrossRef]
- Jarrell, W.M.; Beverly, R.B. Dilution effect in plant nutrition studies. Adv. Agron. 1981, 34, 197–224. [Google Scholar]
- Grifoni, M.; Rosellini, I.; Petruzzelli, G.; Pedron, F.; Franchi, E.; Barbafieri, M. Application of sulphate and cytokinin in assisted arsenic phytoextraction by industrial Cannabis sativa L. Environ. Sci. Pollut. Res. Int. 2021, 28, 47294–47305. [Google Scholar] [CrossRef]
- Franchi, E.; Cosmina, P.; Pedron, F.; Rosellini, I.; Barbafieri, M.; Petruzzelli, G.; Vocciante, M. Improved arsenic phytoextraction by combined use of mobilizing chemicals and autochthonous soil bacteria. Sci. Total Environ. 2019, 655, 328–336. [Google Scholar] [CrossRef]
- Keller, C.; Hammer, D.; Kayser, A.; Richner, W.; Brodbeck, M.; Sennhauser, M. Root development and heavy metal phytoextraction efficiency: Comparison of different plant species in the field. Plant Soil 2003, 249, 67–81. [Google Scholar] [CrossRef]
- Sung, M.; Lee, C.Y.; Lee, S.Z. Combined mild soil washing and compost-assisted phytoremediation in treatment of silt loams contaminated with copper, nickel, and chromium. J. Hazard. Mater. 2011, 190, 744–754. [Google Scholar] [CrossRef]
- Cruz, N.; Ferreira, S.; Cabral, M.; Simões, P.; Marques, R.C. Packaging waste recycling in Europe: Is the industry paying for it? Waste Manag. 2014, 34, 298–308. [Google Scholar] [CrossRef] [Green Version]
- Marques, R.; Simões, P.; Pinto, F. Tariff regulation in the waste sector: An unavoidable future. Waste Manag. 2018, 78, 292–300. [Google Scholar] [CrossRef]
- Vocciante, M.; De Folly D’Auris, A.; Franchi, E.; Petruzzelli, G.; Ferro, S. CO2 footprint analysis of consolidated and innovative technologies in remediation activities. J. Clean. Prod. 2021, 297, 126723. [Google Scholar] [CrossRef]
- Taghipour, H.; Mosaferi, M.; Armanfar, F.; Gaemmagami, S.J. Heavy metals pollution in the soils of suburban areas in big cities: A case study. Int. J. Environ. Sci. Technol. 2013, 10, 243–250. [Google Scholar] [CrossRef]






| B. juncea | Z. mays | |||
|---|---|---|---|---|
| U-Soil | T-Soil | U-Soil | T-Soil | |
| Cd | 0.54 | 0.28 | 0.52 | 0.47 |
| Zn | 0.41 | 0.17 | 0.52 | 0.50 |
| Ni | 0.38 | 0.08 | 0.40 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedron, F.; Grifoni, M.; Barbafieri, M.; Franchi, E.; Vocciante, M.; Petruzzelli, G. Comparative Evaluation of Technologies at a Heavy Metal Contaminated Site: The Role of Feasibility Studies. Environments 2022, 9, 139. https://doi.org/10.3390/environments9110139
Pedron F, Grifoni M, Barbafieri M, Franchi E, Vocciante M, Petruzzelli G. Comparative Evaluation of Technologies at a Heavy Metal Contaminated Site: The Role of Feasibility Studies. Environments. 2022; 9(11):139. https://doi.org/10.3390/environments9110139
Chicago/Turabian StylePedron, Francesca, Martina Grifoni, Meri Barbafieri, Elisabetta Franchi, Marco Vocciante, and Gianniantonio Petruzzelli. 2022. "Comparative Evaluation of Technologies at a Heavy Metal Contaminated Site: The Role of Feasibility Studies" Environments 9, no. 11: 139. https://doi.org/10.3390/environments9110139
APA StylePedron, F., Grifoni, M., Barbafieri, M., Franchi, E., Vocciante, M., & Petruzzelli, G. (2022). Comparative Evaluation of Technologies at a Heavy Metal Contaminated Site: The Role of Feasibility Studies. Environments, 9(11), 139. https://doi.org/10.3390/environments9110139