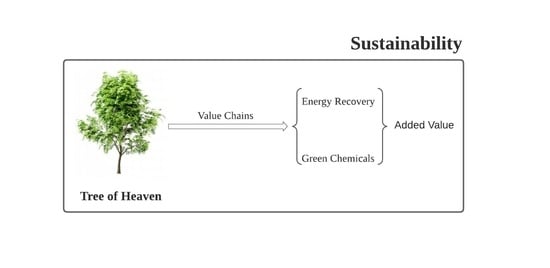
Creation of Value Chains for the Sustainability of Control and Eradication Actions on Ailanthus altissima (Mill.) Swingle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bibliometric Analysis
2.2. Collection and Preparation of A. altissima Samples
2.3. Laboratory Characterization
- ISO 17225-1: 2014—Solid biofuels—Fuel specifications and classes—Part 1: General requirements;
- ISO 16948: 2015—Solid biofuels—Determination of total content of C, H and N;
- ISO 16967: 2015—Solid biofuels—Determination of major elements—Al, Ca, Fe, Mg, P, K, Si, Na and Ti;
- ISO 16968: 2015—Solid biofuels—Determination of minor elements—Ar, Cd, Co, Cr, Cu, Hg, Mn, Mo, Ni, Pb, Sb, V and Zn;
- ISO 16994: 2016—Solid biofuels—Determination of total content of S and Cl;
- ISO 18125: 2017—Solid biofuels—Determination of calorific value;
- ISO 21404: 2020 (en)—Solid biofuels—Determination of ash melting behavior;
- ASTM E870-82 (2019)—Standard Test Methods for Analysis of Wood Fuels (with reference documents: ASTM D1102-84 (2021)—Standard Test Method for Ash in Wood; ASTM E871-82 (2019)—Standard Test Method for Moisture Analysis of Particulate Wood Fuels; ASTM E871-82 (2019)—Standard Test Method for Moisture Analysis of Particulate Wood Fuels)—Determination of proximate analysis by thermogravimetry.
3. Results
3.1. Results of Bibliographic Analyses
3.2. Laboratorial Characterization of the A. altissima Samples
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, J.; Wu, Y.; Zheng, H.; Fu, W.; Reardon, R.; Liu, M. Assessing potential biological control of the invasive plant, tree-of-heaven, Ailanthus altissima. Biocontrol Sci. Technol. 2006, 16, 547–566. [Google Scholar] [CrossRef]
- Sladonja, B.; Sušek, M.; Guillermic, J. Review on invasive tree of heaven (Ailanthus altissima (Mill.) Swingle) conflicting values: Assessment of its ecosystem services and potential biological threat. Environ. Manag. 2015, 56, 1009–1034. [Google Scholar] [CrossRef] [PubMed]
- Kožuharova, E.; Lebanova, H.; Getov, I.; Benbassat, N.; Kochmarov, V. Ailanthus altissima (Mill.) Swingle-a terrible invasive pest in Bulgaria or potential useful medicinal plant. Bothalia J. 2014, 44, 213–230. [Google Scholar]
- Pötzelsberger, E.; Spiecker, H.; Neophytou, C.; Mohren, F.; Gazda, A.; Hasenauer, H. Growing non-native trees in European forests brings benefits and opportunities but also has its risks and limits. Curr. For. Rep. 2020, 6, 339–353. [Google Scholar] [CrossRef]
- Gregory, S.V.; Swanson, F.J.; McKee, W.A.; Cummins, K.W. An ecosystem perspective of riparian zones. BioScience 1991, 41, 540–551. [Google Scholar] [CrossRef]
- Pouyat, R.V.; Yesilonis, I.D.; Golubiewski, N.E. A comparison of soil organic carbon stocks between residential turf grass and native soil. Urban Ecosyst. 2009, 12, 45–62. [Google Scholar] [CrossRef]
- Styger, E.; Rakotondramasy, H.M.; Pfeffer, M.J.; Fernandes, E.C.; Bates, D.M. Influence of slash-and-burn farming practices on fallow succession and land degradation in the rainforest region of Madagascar. Agric. Ecosyst. Environ. 2007, 119, 257–269. [Google Scholar] [CrossRef]
- Motti, R.; Zotti, M.; Bonanomi, G.; Cozzolino, A.; Stinca, A.; Migliozzi, A. Climatic and anthropogenic factors affect Ailanthus altissima invasion in a Mediterranean region. Plant Ecol. 2021, 222, 1347–1359. [Google Scholar] [CrossRef]
- Landenberger, R.E.; Kota, N.L.; McGraw, J.B. Seed dispersal of the non-native invasive tree Ailanthus altissima into contrasting environments. Plant Ecol. 2007, 192, 55–70. [Google Scholar] [CrossRef]
- Rebbeck, J.; Jolliff, J. How long do seeds of the invasive tree, Ailanthus altissima remain viable? For. Ecol. Manag. 2018, 429, 175–179. [Google Scholar] [CrossRef]
- Hierro, J.L.; Callaway, R.M. The ecological importance of allelopathy. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 25–45. [Google Scholar] [CrossRef]
- Kaproth, M.A.; McGraw, J.B. Seed viability and dispersal of the wind-dispersed invasive Ailanthus altissima in aqueous environments. For. Sci. 2008, 54, 490–496. [Google Scholar]
- Meloche, C.; Murphy, S.D. Managing tree-of-heaven (Ailanthus altissima) in parks and protected areas: A case study of Rondeau Provincial Park (Ontario, Canada). Environ. Manag. 2006, 37, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Kota, N.L.; Landenberger, R.E.; McGraw, J.B. Germination and early growth of Ailanthus and tulip poplar in three levels of forest disturbance. Biol. Invasions 2007, 9, 197–211. [Google Scholar] [CrossRef]
- Walker, G.A.; Robertson, M.P.; Gaertner, M.; Gallien, L.; Richardson, D.M. The potential range of Ailanthus altissima (tree of heaven) in South Africa: The roles of climate, land use and disturbance. Biol. Invasions 2017, 19, 3675–3690. [Google Scholar] [CrossRef] [Green Version]
- Albright, T.P.; Chen, H.; Chen, L.; Guo, Q. The ecological niche and reciprocal prediction of the disjunct distribution of an invasive species: The example of Ailanthus altissima. Biol. Invasions 2010, 12, 2413–2427. [Google Scholar] [CrossRef]
- Bullock, J.M.; White, S.M.; Prudhomme, C.; Tansey, C.; Perea, R.; Hooftman, D.A. Modelling spread of British wind-dispersed plants under future wind speeds in a changing climate. J. Ecol. 2012, 100, 104–115. [Google Scholar] [CrossRef] [Green Version]
- Kowarik, I.; Säumel, I. Biological flora of central Europe: Ailanthus altissima (Mill.) swingle. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M.; Leck, M.A. Afterripening pattern during cold stratification of achenes of ten perennial Asteraceae from eastern North America, and evolutionary implication. Plant Species Biol. 1993, 8, 61–65. [Google Scholar] [CrossRef]
- Pan, E.; Bassuk, N. Effects of soil type and compaction on the growth of Ailanthus altissima seedlings. J. Environ. Hortic. 1985, 3, 158–162. [Google Scholar] [CrossRef]
- Day, S.D.; Bassuk, N.L. A review of the effects of soil compaction and amelioration treatments on landscape trees. J. Arboric. 1994, 20, 9–17. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, X.; Caplan, J.S.; Li, M.; Guo, W. Novel plant-soil feedbacks drive adaption of invasive plants to soil legacies of native plants under nitrogen deposition. Plant Soil 2021, 467, 47–65. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Valladares, F. Tolerance to shade, drought, and waterlogging of temperate northern hemisphere trees and shrubs. Ecol. Monogr. 2006, 76, 521–547. [Google Scholar] [CrossRef]
- Radtke, A.; Ambraß, S.; Zerbe, S.; Tonon, G.; Fontana, V.; Ammer, C. Traditional coppice forest management drives the invasion of Ailanthus altissima and Robinia pseudoacacia into deciduous forests. For. Ecol. Manag. 2013, 291, 308–317. [Google Scholar] [CrossRef]
- Arbona, V.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Maintenance of a high photosynthetic performance is linked to flooding tolerance in citrus. Environ. Exp. Bot. 2009, 66, 135–142. [Google Scholar] [CrossRef]
- Yigit, N.; Sevik, H.; Cetin, M.; Kaya, N. Determination of the effect of drought stress on the seed germination in some plant species. In Water Stress in Plants; IntechOpen: London, UK, 2016; pp. 43–62. [Google Scholar]
- Adhikari, P.; Lee, Y.H.; Park, Y.-S.; Hong, S.H. Assessment of the Spatial Invasion Risk of Intentionally Introduced Alien Plant Species (IIAPS) under Environmental Change in South Korea. Biology 2021, 10, 1169. [Google Scholar] [CrossRef]
- Sîrbu, C.; Anastasiu, P.; Urziceanu, M.; Camen-Comănescu, P.; Sîrbu, I.-M.; Popa, A.-M.; Ioja, C.; Gavrilidis, A.-A.; Oprea, A. Invasive alien plant species in Romania of European Union concern. Environ. Socio-Econ. Stud. 2021, 9, 32–44. [Google Scholar] [CrossRef]
- Domina, G. Invasive Aliens in Italy: Enumeration, History, Biology and Their Impact. Invasive Alien Species Obs. Issues Around World 2021, 3, 190–214. [Google Scholar]
- Sohrabi, S.; Pergl, J.; Pyšek, P.; Foxcroft, L.C.; Gherekhloo, J. Quantifying the potential impact of alien plants of Iran using the Generic Impact Scoring System (GISS) and Environmental Impact Classification for Alien Taxa (EICAT). Biol. Invasions 2021, 23, 2435–2449. [Google Scholar] [CrossRef]
- Celesti-Grapow, L.; Ricotta, C. Plant invasion as an emerging challenge for the conservation of heritage sites: The spread of ornamental trees on ancient monuments in Rome, Italy. Biol. Invasions 2021, 23, 1191–1206. [Google Scholar] [CrossRef]
- Brooks, R.K.; Barney, J.N.; Salom, S.M. The invasive tree, Ailanthus altissima, impacts understory nativity, not seedbank nativity. For. Ecol. Manag. 2021, 489, 119025. [Google Scholar] [CrossRef]
- Montagnani, C.; Gentili, R.; Brundu, G.; Caronni, S.; Citterio, S. Accidental Introduction and Spread of Top Invasive Alien Plants in the European Union through Human-Mediated Agricultural Pathways: What Should We Expect? Agronomy 2022, 12, 423. [Google Scholar] [CrossRef]
- Bardsley, D.K.; Edwards-Jones, G. Invasive species policy and climate change: Social perceptions of environmental change in the Mediterranean. Environ. Sci. Policy 2007, 10, 230–242. [Google Scholar] [CrossRef]
- Drucker, H.R.; Brown, C.S.; Stohlgren, T.J. Developing regional invasive species watch lists: Colorado as a case study. Invasive Plant Sci. Manag. 2008, 1, 390–398. [Google Scholar] [CrossRef]
- Castro-Díez, P.; Valle, G.; Gonzalez-Munoz, N.; Alonso, A. Can the life-history strategy explain the success of the exotic trees Ailanthus altissima and Robinia pseudoacacia in Iberian floodplain forests? PLoS ONE 2014, 9, e100254. [Google Scholar] [CrossRef] [Green Version]
- Kheloufi, A.; Mansouri, L.M.; Zerrouni, R.; Abdelhamid, O. Effect of temperature and salinity on germination and seedling establishment of Ailanthus altissima (Mill.) Swingle (Simaroubaceae). Reforesta 2020, 9, 44–53. [Google Scholar] [CrossRef]
- Wickert, K.L.; O’Neal, E.S.; Davis, D.D.; Kasson, M.T. Seed production, viability, and reproductive limits of the invasive Ailanthus altissima (tree-of-heaven) within invaded environments. Forests 2017, 8, 226. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, D.A.; Rauschert, E.S.; Nord, A.N.; Jones, B.P. Forest roads facilitate the spread of invasive plants. Invasive Plant Sci. Manag. 2009, 2, 191–199. [Google Scholar] [CrossRef]
- Motard, E.; Muratet, A.; Clair-Maczulajtys, D.; Machon, N. Does the invasive species Ailanthus altissima threaten floristic diversity of temperate peri-urban forests? Comptes Rendus Biol. 2011, 334, 872–879. [Google Scholar] [CrossRef]
- Knapp, L.B.; Canham, C.D. Invasion of an old-growth forest in New York by Ailanthus altissima: Sapling growth and recruitment in canopy gaps. J. Torrey Bot. Soc. 2000, 127, 307–315. [Google Scholar] [CrossRef]
- Kowarik, I.; Säumel, I. Water dispersal as an additional pathway to invasions by the primarily wind-dispersed tree Ailanthus altissima. Plant Ecol. 2008, 198, 241–252. [Google Scholar] [CrossRef]
- Peñuelas, J.; Sardans, J. Global change and forest disturbances in the Mediterranean basin: Breakthroughs, knowledge gaps, and recommendations. Forests 2021, 12, 603. [Google Scholar] [CrossRef]
- Nunes, L.J.; Raposo, M.A.; Pinto Gomes, C.J. A historical perspective of landscape and human population dynamics in Guimarães (Northern Portugal): Possible implications of rural fire risk in a changing environment. Fire 2021, 4, 49. [Google Scholar] [CrossRef]
- Barudanović, S.; Zečić, E.; Macanović, A.; Duraković, B.; Mašić, E. Invasive alien plant species in global perspectives with special references to Bosnia and Herzegovina. Invasive Alien Species Obs. Issues Around World 2021, 3, 215–252. [Google Scholar]
- Knüsel, S.; Conedera, M.; Rigling, A.; Fonti, P.; Wunder, J. A tree-ring perspective on the invasion of Ailanthus altissima in protection forests. For. Ecol. Manag. 2015, 354, 334–343. [Google Scholar] [CrossRef]
- Gutiérrez-López, M.; Ranera, E.; Novo, M.; Fernández, R.; Trigo, D. Does the invasion of the exotic tree Ailanthus altissima affect the soil arthropod community? The case of a riparian forest of the Henares River (Madrid). Eur. J. Soil Biol. 2014, 62, 39–48. [Google Scholar] [CrossRef]
- DiTomaso, J.M.; Kyser, G.B. Control of Ailanthus altissima using stem herbicide application techniques. Arboric. Urban For. 2007, 33, 55–63. [Google Scholar] [CrossRef]
- Constán-Nava, S.; Bonet, A.; Pastor, E.; Lledó, M.J. Long-term control of the invasive tree Ailanthus altissima: Insights from Mediterranean protected forests. For. Ecol. Manag. 2010, 260, 1058–1064. [Google Scholar] [CrossRef]
- Heisey, R.M.; Heisey, T.K. Herbicidal effects under field conditions of Ailanthus altissima bark extract, which contains ailanthone. Plant Soil 2003, 256, 85–99. [Google Scholar] [CrossRef]
- Burch, P.L.; Zedaker, S.M. Removing the invasive tree Ailanthus altissima and restoring natural cover. Arboric. Urban For. 2003, 29, 18–24. [Google Scholar] [CrossRef]
- Nunes, L.J.; Raposo, M.A.; Meireles, C.I.; Pinto Gomes, C.J.; Ribeiro, N.; Almeida, M. Control of invasive forest species through the creation of a value chain: Acacia dealbata biomass recovery. Environments 2020, 7, 39. [Google Scholar] [CrossRef]
- Byun, C.; Lee, E.J. Ecological application of biotic resistance to control the invasion of an invasive plant, Ageratina altissima. Ecol. Evol. 2017, 7, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Caser, M.; Demasi, S.; Caldera, F.; Dhakar, N.K.; Trotta, F.; Scariot, V. Activity of Ailanthus altissima (Mill.) swingle extract as a potential bioherbicide for sustainable weed management in horticulture. Agronomy 2020, 10, 965. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Almeida, M.; Mouga, T.; Barracosa, P. The weathering ability of higher plants. The case of Ailanthus altissima (Miller) Swingle. Int. Biodeterior. Biodegrad. 1994, 33, 333–343. [Google Scholar] [CrossRef]
- Raposo, M.A.; Nunes, L.J.; Quinto-Canas, R.; del Río, S.; Pardo, F.M.V.; Galveias, A.; Pinto-Gomes, C.J. Prunus lusitanica L.: An endangered plant species relict in the central region of mainland Portugal. Diversity 2021, 13, 359. [Google Scholar] [CrossRef]
- Neto Duarte, L.; Pinto Gomes, C.; Marchante, H.; Marchante, E. Integrating knowledge of ecological succession into invasive alien plant management: A case study from Portugal. Appl. Veg. Sci. 2020, 23, 328–339. [Google Scholar] [CrossRef]
- Ururahy-Rodrigues, A.; Rafael, J.A.; Pujol-Luz, J.R. Temporal distribution of blowflies of forensic importance (Diptera: Calliphoridae), in man-size domestic pigs carcasses, in the Forest Reserve Adolpho Ducke, Manaus, Amazonas, Brazil. EntomoBrasilis 2013, 6, 09–22. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, F.; Ferreira, M. Plant invasions in the rivers of the Iberian Peninsula, south-western Europe: A review. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2013, 147, 1107–1119. [Google Scholar] [CrossRef]
- Aguiar, F.; Ferreira, M.; Moreira, I. Exotic and native vegetation establishment following channelization of a western Iberian river. Regul. Rivers Res. Manag. Int. J. Devoted River Res. Manag. 2001, 17, 509–526. [Google Scholar] [CrossRef]
- Martins, F.; Alegria, C.; Artur, G. Mapping invasive alien Acacia dealbata Link using ASTER multispectral imagery: A case study in central-eastern of Portugal. For. Syst. 2016, 25, e078. [Google Scholar] [CrossRef] [Green Version]
- Brunel, S.; Brundu, G.; Fried, G. Eradication and control of invasive alien plants in the M editerranean B asin: Towards better coordination to enhance existing initiatives. EPPO Bull. 2013, 43, 290–308. [Google Scholar] [CrossRef]
- Sitzia, T.; Campagnaro, T.; Kowarik, I.; Trentanovi, G. Using forest management to control invasive alien species: Helping implement the new European regulation on invasive alien species. Biol. Invasions 2016, 18, 1–7. [Google Scholar] [CrossRef]
- Fernandes, M.; Devy-Vareta, N.; Rangan, H. Plantas exóticas invasoras e instrumentos de gestão territorial. O caso paradigmático do género Acacia em Portugal. Rev. Geogr. Ordenam. Territ. 2013, 1, 83–107. [Google Scholar] [CrossRef] [Green Version]
- Nunes, L.J.; Raposo, M.A.; Meireles, C.I.; Gomes, C.J.P.; Ribeiro, N.; Almeida, M. The Impact of Rural Fires on the Development of Invasive Species: Analysis of a Case Study with Acacia dealbata Link. in Casal do Rei (Seia, Portugal). Environments 2021, 8, 44. [Google Scholar] [CrossRef]
- Santos, A.; Simões, R.; Tavares, M. Variation of some wood macroscopic properties along the stem of Acacia melanoxylon R. Br. adult trees in Portugal. For. Syst. 2013, 22, 463–470. [Google Scholar] [CrossRef]
- Casinovi, C.G.; Ceccherelli, P. On the Structure of Ailanthone. Tetrahedron Lett. 1964, 5, 3991–3997. [Google Scholar] [CrossRef]
- Naora, H.; Furuno, T.; Ishibashi, M.; Tsuyuki, T.; Takahashi, T.; Itai, A.; Iitaka, Y.; Polonsky, J. On the Structure of Ailanthone, A Bitter Principle from Ailanthus altissima. Chem. Lett. 1982, 11, 661–662. [Google Scholar] [CrossRef]
- Casinovi, C.G.; Fardella, G.; Grandolini, G.; Burinato, C. Anti-Amebic Property of Some Derivatives of Ailanthone and Quassin. Farm.-Ed. Sci. 1981, 36, 116–122. [Google Scholar]
- Lin, L.J.; Peiser, G.; Ying, B.P.; Mathias, K.; Karasina, F.; Wang, Z.; Itatani, J.; Green, L.; Hwang, Y.S. Identification of Plant-Growth Inhibitory Principles in Ailanthus altissima and Castela tortuosa. J. Agric. Food Chem. 1995, 43, 1708–1711. [Google Scholar] [CrossRef]
- Heisey, R.M. Identification of an allelopathic compound from Ailanthus altissima (Simaroubaceae) and characterization of its herbicidal activity. Am. J. Bot. 1996, 83, 192–200. [Google Scholar] [CrossRef]
- De Feo, V.; De Martino, L.; Quaranta, E.; Pizza, C. Isolation of phytotoxic compounds from tree-of-heaven (Ailanthus altissima Swingle). J. Agric. Food Chem. 2003, 51, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Pedersini, C.; Bergamin, M.; Aroulmoji, V.; Baldini, S.; Picchio, R.; Pesce, P.G.; Ballarin, L.; Murano, E. Herbicide Activity of Extracts from Ailanthus altissima (Simaroubaceae). Nat. Prod. Commun. 2011, 6, 593–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehorska, R.; Jamnig, J.; Lernbeiss, J.; Drescher, A.; Muller, M.; Pfeifhofer, H.W. Growth Stimulating Effects of Ailanthus altissima Root and Leaf Extractives on Radicle and Hypocotyl Growth of Garden Cress (Lepidium sativum) and a Possible Evidence for Growth Inhibiting Root Exudates. Phyton-Ann. REI Bot. 2016, 56, 49–59. [Google Scholar] [CrossRef]
- Honda, T.; Imao, K.; Inoueshiraishi, M.; Nakatsuka, N.; Tatsuoka, T.; Nakanishi, T.; Noguchi, T. Studies on Antitumor-Activity of Quassinoids—Synthesis and Antitumor-Activity of Novel Ailanthone Analogs. J. Pharm.-Dyn. 1987, 10, S61. [Google Scholar]
- Kato, T.; Suzumura, Y.; Fukushima, M.; Honda, T.; Nakanishi, T.; Noguchi, T. Antitumor-Activity of Novel Ailanthone Derivatives Invitro and Invivo. Anticancer Res. 1988, 8, 573–580. [Google Scholar]
- Zhuo, Z.J.; Hu, J.Y.; Yang, X.L.; Chen, M.F.; Lei, X.P.; Deng, L.J.; Yao, N.; Peng, Q.L.; Chen, Z.S.; Ye, W.C.; et al. Ailanthone Inhibits Huh7 Cancer Cell Growth via Cell Cycle Arrest and Apoptosis In Vitro and In Vivo. Sci. Rep. 2015, 5, 16185. [Google Scholar] [CrossRef] [Green Version]
- He, Y.D.; Peng, S.H.; Wang, J.H.; Chen, H.; Cong, X.N.; Chen, A.; Hu, M.C.; Qin, M.; Wu, H.G.; Gao, S.M.; et al. Ailanthone targets p23 to overcome MDV3100 resistance in castration-resistant prostate cancer. Nat. Commun. 2016, 7, 13122. [Google Scholar] [CrossRef]
- Chen, Y.X.; Zhu, L.; Yang, X.; Wei, C.; Chen, C.R.; He, Y.; Ji, Z.N. Ailanthone induces G(2)/M cell cycle arrest and apoptosis of SGC-7901 human gastric cancer cells. Mol. Med. Rep. 2017, 16, 6821–6827. [Google Scholar] [CrossRef] [Green Version]
- Ni, Z.Y.; Yao, C.; Zhu, X.W.; Gong, C.Y.; Xu, Z.H.; Wang, L.X.; Li, S.Y.; Zou, C.P.; Zhu, S.G. Ailanthone inhibits non-small cell lung cancer cell growth through repressing DNA replication via downregulating RPA1. Br. J. Cancer 2017, 117, 1621–1630. [Google Scholar] [CrossRef]
- Peng, S.H.; Yi, Z.F.; Liu, M.Y. Ailanthone: A new potential drug for castration-resistant prostate cancer. Chin. J. Cancer 2017, 36, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.X.; Lu, Y.J.; Li, H.; Sun, L.X.; Yang, N.; Zhao, M.Z.; Zhang, M.L.; Shi, Q.W. Antitumor activity of the Ailanthus altissima bark phytochemical ailanthone against breast cancer MCF-7 cells. Oncol. Lett. 2018, 15, 6022–6028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Chen, C.R.; Cheng, Y.X.; Zhu, L.; Wang, Y.; Luo, C.; He, Y.; Yang, Z.M.; Ji, Z.M. Ailanthone induces autophagic and apoptotic cell death in human promyelocytic leukemia HL-60 cells. Oncol. Lett. 2018, 16, 3569–3576. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.Z.; Sun, D.Z.; Jiang, F. Ailanthone Promotes Human Vestibular Schwannoma Cell Apoptosis and Autophagy by Downregulation of miR-21. Oncol. Res. 2018, 26, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Daga, M.; Pizzimenti, S.; Dianzani, C.; Cucci, M.A.; Cavalli, R.; Grattarola, M.; Ferrara, B.; Scariot, V.; Trotta, F.; Barrera, G. Ailanthone inhibits cell growth and migration of cisplatin resistant bladder cancer cells through down-regulation of Nrf2, YAP, and c-Myc expression. Phytomedicine 2019, 56, 156–164. [Google Scholar] [CrossRef]
- Gao, W.; Ge, S.K.; Sun, J.Y. Ailanthone exerts anticancer effect by up-regulating miR-148a expression in MDA-MB-231 breast cancer cells and inhibiting proliferation, migration and invasion. Biomed. Pharmacother. 2019, 109, 1062–1069. [Google Scholar] [CrossRef]
- Liu, W.J.; Liu, X.N.; Pan, Z.H.; Wang, D.; Li, M.J.; Chen, X.Y.; Zhou, L.; Xu, M.L.; Li, D.F.; Zheng, Q.S. Ailanthone Induces Cell Cycle Arrest and Apoptosis in Melanoma B16 and A375 Cells. Biomolecules 2019, 9, 275, Retraction in Biomolecules 2020, 10, 627. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, C.Z.; Min, D.J. Ailanthone up-regulates miR-449a to restrain acute myeloid leukemia cells growth, migration and invasion. Exp. Mol. Pathol. 2019, 108, 114–120. [Google Scholar] [CrossRef]
- Tang, S.W.; Ma, X.R.; Lu, J.; Zhang, Y.J.; Liu, M.Y.; Wang, X. Preclinical toxicology and toxicokinetic evaluation of ailanthone, a natural product against castration-resistant prostate cancer, in mice. Fitoterapia 2019, 136, 104161. [Google Scholar] [CrossRef]
- Bailly, C. Anticancer properties and mechanism of action of the quassinoid ailanthone. Phytother. Res. 2020, 34, 2203–2213. [Google Scholar] [CrossRef]
- Cucci, M.A.; Grattarola, M.; Dianzani, C.; Damia, G.; Ricci, F.; Roetto, A.; Trotta, F.; Barrera, G.; Pizzimenti, S. Ailanthone increases oxidative stress in CDDP-resistant ovarian and bladder cancer cells by inhibiting of Nrf2 and YAP expression through a post-translational mechanism. Free Radic. Biol. Med. 2020, 150, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.X.; Yu, X.C.; Hang, C.; Gao, K.J.; Lao, X.F.; Jia, Y.T.; Yan, Z.L. Ailanthone: A novel potential drug for treating human cancer. Oncol. Lett. 2020, 20, 1489–1503. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, C.; Wang, H.M.; Chen, S.N.; Ma, D.H.; Tao, Y.; Wang, X.Y.; Luan, Y.H.; Wang, T.D.; Shi, Y.; et al. Analysis of Long Noncoding RNAs in Aila-Induced Non-Small Cell Lung Cancer Inhibition. Front. Oncol. 2021, 11, 652567. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Li, H.F.; Wang, X.K.; Li, W.G.; Su, Q.; Xiao, X.; Hao, T.F.; Chen, W.; Zhang, Y.W.; Zhang, H.Y.; et al. Ailanthus altissima-derived Ailanthone enhances Gastric Cancer Cell Apoptosis by Inducing the Repression of Base Excision Repair by Downregulating p23 Expression. Int. J. Biol. Sci. 2021, 17, 2811–2825. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.X.; Yu, X.C.; Yan, Z.L. Ailanthone suppresses the activity of human colorectal cancer cells through the STAT3 signaling pathway. Int. J. Mol. Med. 2022, 49, 11. [Google Scholar] [CrossRef]
- Rahman, S.; Fukamiya, N.; Okano, M.; Tagahara, K.; Lee, K.H. Anti-tuberculosis activity of quassinoids. Chem. Pharm. Bull. 1997, 45, 1527–1529. [Google Scholar] [CrossRef] [Green Version]
- Okunade, A.L.; Bikoff, R.E.; Casper, S.J.; Oksman, A.; Goldberg, D.E.; Lewis, W.H. Antiplasmodial activity of extracts and quassinoids isolated from seedlings of Ailanthus altissima (Simaroubaceae). Phytother. Res. 2003, 17, 675–677. [Google Scholar] [CrossRef]
- Han, F.; Liu, G.Q.; Sun, C.F.; Wei, J.N. Ailanthone reverses multidrug resistance by inhibiting the P-glycoprotein-mediated efflux in resistant K562/A02 cells. Cell. Mol. Biol. 2018, 64, 55–61. [Google Scholar] [CrossRef]
- Hou, S.Z.; Cheng, Z.M.; Wang, W.L.; Wang, X.D.; Wu, Y.B. Ailanthone exerts an antitumor function on the development of human lung cancer by upregulating microRNA-195. J. Cell. Biochem. 2019, 120, 10444–10451, Retraction in J. Cell. Biochem. 2021, 122, 489. [Google Scholar] [CrossRef]
- Kong, D.L.; Ying, B.D.; Zhang, J.R.; Ying, H.L. The anti-osteosarcoma property of ailanthone through regulation of miR-126/VEGF-A axis. Artif. Cell. Nanomed. Biotechnol. 2020, 48, 1254, Retraction of Artif. Cell. Nanomed. Biotechnol. 2019, 47, 3913. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Caldera, F.; Dhakar, N.K.; Vidotto, F.; Trotta, F.; Scariot, V. Functionalized dextrin-based nanosponges as effective carriers for the herbicide ailanthone. Ind. Crops Prod. 2021, 164, 113346. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Ma, S.B.; Zhao, Q.Q.; Wu, J.S.; Duan, L.R.; Xie, Y.H.; Wang, S.W. Traditional uses, phytochemistry, and pharmacology of Ailanthus altissima (Mill.) Swingle bark: A comprehensive review. J. Ethnopharmacol. 2021, 275, 114121. [Google Scholar] [CrossRef]
- Novak, M.; Novak, N.; Milinovic, B. Differences in allelopathic effect of tree of heaven root extracts and isolated ailanthone on test-species. J. Cent. Eur. Agric. 2021, 22, 611–622. [Google Scholar] [CrossRef]
- Alidou-Arzika, I.; Lebrun, M.; Miard, F.; Nandillon, R.; BAYÇU, G.; Bourgerie, S.; Morabito, D. Assessment of compost and three biochars associated with Ailanthus altissima (Miller) Swingle for lead and arsenic stabilization in a post-mining Technosol. Pedosphere 2021, 31, 944–953. [Google Scholar] [CrossRef]
- Nunes, L.J.; Raposo, M.A.; Meireles, C.I.; Pinto Gomes, C.J.; Almeida Ribeiro, N. Carbon Sequestration Potential of Forest Invasive Species: A Case Study with Acacia dealbata Link. Resources 2021, 10, 51. [Google Scholar] [CrossRef]
- Nunes, L.J.; Rodrigues, A.M.; Loureiro, L.M.; Sá, L.C.; Matias, J.C. Energy Recovery from Invasive Species: Creation of Value Chains to Promote Control and Eradication. Recycling 2021, 6, 21. [Google Scholar] [CrossRef]
- Nunes, L.J.; Loureiro, L.M.; Sá, L.C.; Matias, J.C.; Ferraz, A.I.; Rodrigues, A.C. Energy Recovery of Agricultural Residues: Incorporation of Vine Pruning in the Production of Biomass Pellets with ENplus® Certification. Recycling 2021, 6, 28. [Google Scholar] [CrossRef]
- Nunes, L.J.; Raposo, M.A.; Meireles, C.I.; Gomes, C.J.P.; Ribeiro, N.; Almeida, M. Energy Recovery of Shrub Species as a Path to Reduce the Risk of Occurrence of Rural Fires: A Case Study in Serra da Estrela Natural Park (Portugal). Fire 2021, 4, 33. [Google Scholar] [CrossRef]
- Nunes, L.J. Characterization of Cytisus striatus (Hill) Rothm.: Waste Biomass Energy Recovery as a Measure to Reduce the Risk of Rural Fires. Recycling 2021, 6, 36. [Google Scholar] [CrossRef]
- Samsatli, S.; Samsatli, N.J. The role of renewable hydrogen and inter-seasonal storage in decarbonising heat—Comprehensive optimisation of future renewable energy value chains. Appl. Energy 2019, 233, 854–893. [Google Scholar] [CrossRef]
- Reigosa, M.J.; Pedrol, N.; González, L. Allelopathy: A Physiological Process with Ecological Implications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Putnam, A.R.; Duke, W.B. Allelopathy in agroecosystems. Annu. Rev. Phytopathol. 1978, 16, 431–451. [Google Scholar] [CrossRef]
- Heisey, R.M. Evidence for allelopathy by tree-of-heaven (Ailanthus altissima). J. Chem. Ecol. 1990, 16, 2039–2055. [Google Scholar] [CrossRef]
- Heisey, R.M. Allelopathy and the secret life of Ailanthus altissima. Arnoldia 1997, 57, 28–36. [Google Scholar]
- Heisey, R.M. Allelopathic and herbicidal effects of extracts from tree of heaven (Ailanthus altissima). Am. J. Bot. 1990, 77, 662–670. [Google Scholar] [CrossRef]
- Small, C.J.; White, D.C.; Hargbol, B. Allelopathic influences of the invasive Ailanthus altissima on a native and a non-native herb1, 2. J. Torrey Bot. Soc. 2010, 137, 366–372. [Google Scholar] [CrossRef]
- Meng, P.; Pei, H.; Hu, W.; Liu, Z.; Li, X.; Xu, H. Allelopathic effects of Ailanthus altissima extracts on Microcystis aeruginosa growth, physiological changes and microcystins release. Chemosphere 2015, 141, 219–226. [Google Scholar] [CrossRef]






| Keywords | Nr. of Documents |
|---|---|
| “Ailanthus” and “altissima” | 772 |
| “Ailanthone” | 67 |
| “Ailanthus” and “altissima” and “ecosystem” and “impacts” | 60 |
| “Ailanthus” and “altissima” and “invasive” and “behavior” | 49 |
| “Ailanthus” and “altissima” and “allelopathy” | 12 |
| “Ailanthus” and “altissima” and “control” and “actions” | 5 |
| “Ailanthus” and “altissima” and “value” and “chains” | 1 |
| C (%) | H (%) | N (%) | O (%) | S (%) | Cl (%) | |
|---|---|---|---|---|---|---|
| Location 1 | 47.30 | 5.94 | 0.511 | 46.25 | 0.0258 | 0.0912 |
| Location 2 | 47.56 | 5.82 | 0.498 | 46.12 | 0.0284 | 0.1034 |
| Location 3 | 47.71 | 5.12 | 0.523 | 46.65 | 0.0225 | 0.1051 |
| Average | 47.52 | 5.63 | 0.51 | 46.34 | 0.0225 | 0.0999 |
| Standard deviation | 0.21 | 0.44 | 0.01 | 0.27 | 0.003 | 0.008 |
| LHV (MJ/kg) | HHV (MJ/kg) | |
|---|---|---|
| Location 1 | 20.68 | 21.97 |
| Location 2 | 21.59 | 22.88 |
| Location 3 | 21.36 | 21.84 |
| Average | 21.21 | 22.23 |
| Standard deviation | 0.473 | 0.567 |
| Major Elements | Al (mg/kg) | Ca (mg/kg) | Fe (mg/kg) | Mg (mg/kg) | P (mg/kg) | K (mg/kg) | Si (mg/kg) | Na (mg/kg) | Ti (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|
| Location 1 | 43.0 | 7108.4 | 326.4 | 1639.4 | 1980.0 | 6844.4 | 41.2 | 178.9 | 3.7 |
| Location 2 | 27.9 | 8083.2 | 198.0 | 1640.6 | 2173.4 | 6238.5 | 18.3 | 192.5 | 4.5 |
| Location 3 | 12.3 | 6006.8 | 168.0 | 1364.2 | 1816.1 | 5368.7 | 27.9 | 78.2 | 3.1 |
| Average | 27.7 | 7066.2 | 230.8 | 1549.7 | 1984.8 | 6150.6 | 29.1 | 149.9 | 3.8 |
| Standard deviation | 15.4 | 1038.9 | 84.2 | 156.4 | 178.9 | 741.9 | 11.5 | 62.4 | 0.7 |
| Minor Elements | As (mg/kg) | Cd (mg/kg) | Co (mg/kg) | Cr (mg/kg) | Cu (mg/kg) | Mn (mg/kg) | Ni (mg/kg) | Pb (mg/kg) | Zn (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|
| Location 1 | 1.9 | 0.2 | 8.9 | 18.6 | 10.2 | <0.01 | 10.1 | 3.1 | 25.9 |
| Location 2 | 1.2 | 0.2 | 6.3 | 13.5 | 12.7 | <0.01 | 9.8 | 3.1 | 26.3 |
| Location 3 | 1.6 | 0.1 | 8.0 | 17.3 | 8.8 | <0.01 | 11.2 | 3.5 | 21.6 |
| Average | 1.6 | 0.2 | 7.9 | 16.5 | 10.6 | - | 10.4 | 3.2 | 24.6 |
| Standard deviation | 0.3 | 0.1 | 1.1 | 2.6 | 2.0 | - | 0.7 | 0.2 | 2.6 |
| Parameter | Units | ENPlus® Reference Values | P. pinaster | A. altissima | ||
|---|---|---|---|---|---|---|
| A1 | A2 | B | ||||
| Moisture | % | ≤10.0 | 6.42 | 8.29 | ||
| Ashes | % | ≤0.7 | ≤1.2 | ≤2 | 0.62 | 2.96 |
| LHV | MJ/kg | ≥16.5 | 17.87 | 21.21 | ||
| N | % | ≤0.3 | ≤0.5 | ≤1.0 | 0.08 | 0.511 |
| S | % | ≤0.04 | ≤0.05 | 0.0045 | 0.026 | |
| Cl | % | ≤0.02 | ≤0.03 | 0.02 | 0.100 | |
| Tdeformation | °C | ≥1200 | ≥1100 | 1215 | 1138 | |
| As | % | ≤1 | 0.94 | 1.55 | ||
| Cd | % | ≤0.5 | 0.34 | 0.18 | ||
| Cr | % | ≤10 | 1.99 | 16.46 | ||
| Cu | % | ≤10 | 3.55 | 10.56 | ||
| Pb | % | ≤10 | 0.71 | 3.23 | ||
| Hg | % | ≤0.1 | ≤0.01 | ≤0.01 | ||
| Ni | % | ≤10 | 1.08 | 10.36 | ||
| Zn | % | ≤100 | 8.08 | 24.58 | ||
| Operation | Average Value (€/t) |
|---|---|
| Cut and branches cleaning | 35.00 |
| Retrieval and extraction | 27.50 |
| Transport with loading and unloading | 36.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, L.J.R. Creation of Value Chains for the Sustainability of Control and Eradication Actions on Ailanthus altissima (Mill.) Swingle. Environments 2022, 9, 64. https://doi.org/10.3390/environments9050064
Nunes LJR. Creation of Value Chains for the Sustainability of Control and Eradication Actions on Ailanthus altissima (Mill.) Swingle. Environments. 2022; 9(5):64. https://doi.org/10.3390/environments9050064
Chicago/Turabian StyleNunes, Leonel J. R. 2022. "Creation of Value Chains for the Sustainability of Control and Eradication Actions on Ailanthus altissima (Mill.) Swingle" Environments 9, no. 5: 64. https://doi.org/10.3390/environments9050064
APA StyleNunes, L. J. R. (2022). Creation of Value Chains for the Sustainability of Control and Eradication Actions on Ailanthus altissima (Mill.) Swingle. Environments, 9(5), 64. https://doi.org/10.3390/environments9050064