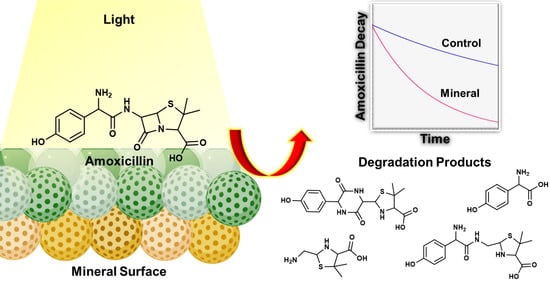
Heterogeneous Photocatalysis of Amoxicillin under Natural Conditions and High-Intensity Light: Fate, Transformation, and Mineralogical Impacts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Particle Characterization
2.3. Degradation Studies
2.3.1. Batch Reactor Studies
2.3.2. Liquid Chromatography-Mass Spectroscopy (LC-MS)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Particle Characterization
3.2. Photo-Degradation Studies
3.3. Amoxicillin Degradation with Terrestrial Solar Spectrum (AM 1.5G Filter)
3.4. Amoxicillin Degradation Mechanism under Terrestrial Solar Radiation
3.5. Amoxicillin Degradation under Dark Conditions
3.6. Amoxicillin Degradation with High-Intensity Light (AM 0 Filter)
3.7. Amoxicillin Degradation Mechanisms with High-Intensity Light
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.; Kim, C.; Liu, X.; Lee, S.; Kho, Y.; Kim, W.-K.; Kim, P.; Choi, K. Ecological Risk Assessment of Amoxicillin, Enrofloxacin, and Neomycin: Are Their Current Levels in the Freshwater Environment Safe? Toxics 2021, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Litskas, V.D.; Karamanlis, X.N.; Prousali, S.P.; Koveos, D.S. Effects of the Antibiotic Amoxicillin on Key Species of the Terrestrial Environment. Bull. Environ. Contam. Toxicol. 2018, 100, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Kim, W.G.; Yoon, Y.; Kang, J.-W.; Hong, Y.M.; Kim, H.W. Removal of amoxicillin by UV and UV/H2O2 processes. Sci. Total Environ. 2012, 420, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, R.; Caprio, V.; Ciniglia, C.; de Champdoré, M.; Lo Giudice, R.; Marotta, R.; Zuccato, E. Antibiotics in the Environment: Occurrence in Italian STPs, Fate, and Preliminary Assessment on Algal Toxicity of Amoxicillin. Environ. Sci. Technol. 2004, 38, 6832–6838. [Google Scholar] [CrossRef] [PubMed]
- Broccoli, A.; Anselmi, S.; Cavallo, A.; Ferrari, V.; Prevedelli, D.; Pastorino, P.; Renzi, M. Ecotoxicological effects of new generation pollutants (nanoparticles, amoxicillin and white musk) on freshwater and marine phytoplankton species. Chemosphere 2021, 279, 130623. [Google Scholar] [CrossRef]
- Sun, X.; Qin, Y.; Zhou, W. Degradation of amoxicillin from water by ultrasound-zero-valent iron activated sodium persulfate. Sep. Purif. Technol. 2021, 275, 119080. [Google Scholar] [CrossRef]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Tetracycline adsorption on kaolinite: pH, metal cations and humic acid effects. Ecotoxicology 2011, 20, 1141–1147. [Google Scholar] [CrossRef]
- Xu, H.; Cooper, W.J.; Jung, J.; Song, W. Photosensitized degradation of amoxicillin in natural organic matter isolate solutions. Water Res. 2011, 45, 632–638. [Google Scholar] [CrossRef]
- Pereira, J.H.O.S.; Reis, A.C.; Homem, V.; Silva, J.A.; Alves, A.; Borges, M.T.; Boaventura, R.A.R.; Vilar, V.J.P.; Nunes, O.C. Solar photocatalytic oxidation of recalcitrant natural metabolic by-products of amoxicillin biodegradation. Water Res. 2014, 65, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Lamm, A.; Gozlan, I.; Rotstein, A.; Avisar, D. Detection of amoxicillin-diketopiperazine-2′, 5′ in wastewater samples. J. Environ. Sci. Health Part A 2009, 44, 1512–1517. [Google Scholar] [CrossRef]
- Timm, A.; Borowska, E.; Majewsky, M.; Merel, S.; Zwiener, C.; Bräse, S.; Horn, H. Photolysis of four β-lactam antibiotics under simulated environmental conditions: Degradation, transformation products and antibacterial activity. Sci. Total Environ. 2019, 651, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.V.; Shriwastav, A. Degradation of amoxicillin with sono, photo, and sonophotocatalytic oxidation under low-frequency ultrasound and visible light. Environ. Res. 2021, 200, 111515. [Google Scholar] [CrossRef] [PubMed]
- Ebele, A.J.; Oluseyi, T.; Drage, D.S.; Harrad, S.; Abou-Elwafa Abdallah, M. Occurrence, seasonal variation and human exposure to pharmaceuticals and personal care products in surface water, groundwater and drinking water in Lagos State, Nigeria. Emerg. Contam. 2020, 6, 124–132. [Google Scholar] [CrossRef]
- Gozlan, I.; Rotstein, A.; Avisar, D. Amoxicillin-degradation products formed under controlled environmental conditions: Identification and determination in the aquatic environment. Chemosphere 2013, 91, 985–992. [Google Scholar] [CrossRef]
- Pérez-Parada, A.; Agüera, A.; Gómez-Ramos, M.d.M.; García-Reyes, J.F.; Heinzen, H.; Fernández-Alba, A.R. Behavior of amoxicillin in wastewater and river water: Identification of its main transformation products by liquid chromatography/electrospray quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 731–742. [Google Scholar] [CrossRef]
- Liu, N.; Han, H.; Yin, H.; Han, L.; Huang, G. Variations in the fate and risk analysis of amoxicillin and its degradation products during pig manure aerobic composting. J. Hazard. Mater. 2018, 346, 234–241. [Google Scholar] [CrossRef]
- Chen, H.; Nanayakkara, C.E.; Grassian, V.H. Titanium Dioxide Photocatalysis in Atmospheric Chemistry. Chem. Rev. 2012, 112, 5919–5948. [Google Scholar] [CrossRef]
- Katal, R.; Masudy-Panah, S.; Tanhaei, M.; Farahani, M.H.D.A.; Jiangyong, H. A review on the synthesis of the various types of anatase TiO2 facets and their applications for photocatalysis. Chem. Eng. J. 2020, 384, 123384. [Google Scholar] [CrossRef]
- Kelleher, B.; O’dwyer, T. Intercalation of benzamide into expanded kaolinite under ambient environmental conditions. Clays Clay Miner 2002, 50, 331–335. [Google Scholar] [CrossRef]
- Scott, A.M.; Burns, E.A.; Hill, F.C. Theoretical study of adsorption of nitrogen-containing environmental contaminants on kaolinite surfaces. J. Mol. Model. 2014, 20, 2373. [Google Scholar] [CrossRef]
- Scott, A.M.; Burns, E.A.; Lafferty, B.J.; Hill, F.C. Theoretical predictions of thermodynamic parameters of adsorption of nitrogen containing environmental contaminants on kaolinite. J. Mol. Model. 2015, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.B.; Gadi, R.; Kalra, S. Synthesis and characterization of novel nanocomposite by using kaolinite and carbon nanotubes. Appl. Clay Sci. 2018, 155, 30–36. [Google Scholar] [CrossRef]
- Benacherine, M.e.m.; Debbache, N.; Ghoul, I.; Mameri, Y. Heterogeneous photoinduced degradation of amoxicillin by Goethite under artificial and natural irradiation. J. Photochem. Photobiol. A Chem. 2017, 335, 70–77. [Google Scholar] [CrossRef]
- Bogialli, S.; Capitolino, V.; Curini, R.; Di Corcia, A.; Nazzari, M.; Sergi, M. Simple and Rapid Liquid Chromatography−Tandem Mass Spectrometry Confirmatory Assay for Determining Amoxicillin and Ampicillin in Bovine Tissues and Milk. J. Agric. Food Chem. 2004, 52, 3286–3291. [Google Scholar] [CrossRef]
- Yang, C.-W.; Liu, C.; Chang, B.-V. Biodegradation of Amoxicillin, Tetracyclines and Sulfonamides in Wastewater Sludge. Water 2020, 12, 2147. [Google Scholar] [CrossRef]
- Khan, S.A.; Siddiqui, M.F.; Khan, T.A. Synthesis of Poly(methacrylic acid)/Montmorillonite Hydrogel Nanocomposite for Efficient Adsorption of Amoxicillin and Diclofenac from Aqueous Environment: Kinetic, Isotherm, Reusability, and Thermodynamic Investigations. ACS Omega 2020, 5, 2843–2855. [Google Scholar] [CrossRef]
- Hettiarachchi, E.; Rubasinghege, G. Mechanistic Study on Iron Solubility in Atmospheric Mineral Dust Aerosol: Roles of Titanium, Dissolved Oxygen, and Solar Flux in Solutions Containing Different Acid Anions. ACS Earth Space Chem. 2020, 4, 101–111. [Google Scholar] [CrossRef]
- Hettiarachchi, E.; Hurab, O.; Rubasinghege, G. Atmospheric Processing and Iron Mobilization of Ilmenite: Iron-Containing Ternary Oxide in Mineral Dust Aerosol. J. Phys. Chem. A 2018, 122, 1291–1302. [Google Scholar] [CrossRef]
- Rubasinghege, G.; Gurung, R.; Rijal, H.; Maldonado-Torres, S.; Chan, A.; Acharya, S.; Rogelj, S.; Piyasena, M. Abiotic degradation and environmental toxicity of ibuprofen: Roles of mineral particles and solar radiation. Water Res. 2018, 131, 22–32. [Google Scholar] [CrossRef]
- Wahyuni, E.; Yulikayani, P.; Aprilita, N. Enhancement of visible-light photocatalytic activity of Cu-doped TiO2 for photodegradation of amoxicillin in water. J. Mater. Environ. Sci. 2020, 11, 670–683. [Google Scholar]
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekoukoulotakis, N.P.; Venieri, D.; Mantzavinos, D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J. Environ. Manage. 2012, 98, 168–174. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 2010, 252, 46–52. [Google Scholar] [CrossRef]
- Moreira, N.F.F.; Orge, C.A.; Ribeiro, A.R.; Faria, J.L.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. Fast mineralization and detoxification of amoxicillin and diclofenac by photocatalytic ozonation and application to an urban wastewater. Water Res. 2015, 87, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Bergamonti, L.; Bergonzi, C.; Graiff, C.; Lottici, P.P.; Bettini, R.; Elviri, L. 3D printed chitosan scaffolds: A new TiO2 support for the photocatalytic degradation of amoxicillin in water. Water Res. 2019, 163, 114841. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Feng, L.; Cheng, X.; Chen, C.; Zhang, L. Photodegradation of amoxicillin in aqueous solution under simulated irradiation: Influencing factors and mechanisms. Water Sci. Technol. 2013, 67, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S.; Yoshimura, C. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: A critical review of recent literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef]
- Çağlar Yılmaz, H.; Akgeyik, E.; Bougarrani, S.; El Azzouzi, M.; Erdemoğlu, S. Photocatalytic degradation of amoxicillin using Co-doped TiO2 synthesized by reflux method and monitoring of degradation products by LC–MS/MS. J. Dispers. Sci. Technol 2020, 41, 414–425. [Google Scholar] [CrossRef]
- Li, Q.; Jia, R.; Shao, J.; He, Y. Photocatalytic degradation of amoxicillin via TiO2 nanoparticle coupling with a novel submerged porous ceramic membrane reactor. J. Clean. Prod. 2019, 209, 755–761. [Google Scholar] [CrossRef]
- Lai, W.W.-P.; Hsu, M.-H.; Lin, A.Y.-C. The role of bicarbonate anions in methotrexate degradation via UV/TiO2: Mechanisms, reactivity and increased toxicity. Water Res. 2017, 112, 157–166. [Google Scholar] [CrossRef]
- Tan, T.-Y.; Zeng, Z.-T.; Zeng, G.-M.; Gong, J.-L.; Xiao, R.; Zhang, P.; Song, B.; Tang, W.-W.; Ren, X.-Y. Electrochemically enhanced simultaneous degradation of sulfamethoxazole, ciprofloxacin and amoxicillin from aqueous solution by multi-walled carbon nanotube filter. Sep. Purif. Technol. 2020, 235, 116167. [Google Scholar] [CrossRef]
- Aksu Demirezen, D.; Yıldız, Y.Ş.; Demirezen Yılmaz, D. Amoxicillin degradation using green synthesized iron oxide nanoparticles: Kinetics and mechanism analysis. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100219. [Google Scholar] [CrossRef]
- Olama, N.; Dehghani, M.; Malakootian, M. The removal of amoxicillin from aquatic solutions using the TiO2/UV-C nanophotocatalytic method doped with trivalent iron. Appl. Water Sci. 2018, 8, 97. [Google Scholar] [CrossRef]
- Zhao, J.; Qin, X.; Wang, J.; He, M. Effect of Mg(II) and Na(I) Doping on the Electronic Structure and Mechanical Properties of Kaolinite. Minerals 2020, 10, 368. [Google Scholar] [CrossRef] [Green Version]
- Tombácz, E.; Szekeres, M. Surface charge heterogeneity of kaolinite in aqueous suspension in comparison with montmorillonite. Appl. Clay Sci. 2006, 34, 105–124. [Google Scholar] [CrossRef]
- Li, X.; Shen, T.; Wang, D.; Yue, X.; Liu, X.; Yang, Q.; Cao, J.; Zheng, W.; Zeng, G. Photodegradation of amoxicillin by catalyzed Fe3+/H2O2 process. J. Environ. Sci. 2012, 24, 269–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Zhong, Y.; Lim, T.-T. Comparison of amoxicillin photodegradation in the UV/H2O2 and UV/persulfate systems: Reaction kinetics, degradation pathways, and antibacterial activity. Chem. Eng. J. 2019, 372, 420–428. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Z.; Dong, S.; Zhou, D. Simultaneous elimination of amoxicillin and antibiotic resistance genes in activated sludge process: Contributions of easy-to-biodegrade food. Sci. Total Environ. 2020, 764, 142907. [Google Scholar] [CrossRef]
- Trovó, A.G.; Pupo Nogueira, R.F.; Agüera, A.; Fernandez-Alba, A.R.; Malato, S. Degradation of the antibiotic amoxicillin by photo-Fenton process—Chemical and toxicological assessment. Water Res. 2011, 45, 1394–1402. [Google Scholar] [CrossRef] [Green Version]
- Busto, R.V.; Roberts, J.; Hunter, C.; Escudero, A.; Helwig, K.; Coelho, L.H.G. Mechanistic and ecotoxicological studies of amoxicillin removal through anaerobic degradation systems. Ecotoxicol. Environ. Saf. 2020, 192, 110207. [Google Scholar] [CrossRef]
- Nägele, E.; Moritz, R. Structure elucidation of degradation products of the antibiotic amoxicillin with ion trap MSn and accurate mass determination by ESI TOF. J. Am. Soc. Mass Spectrom. 2005, 16, 1670–1676. [Google Scholar] [CrossRef] [Green Version]





| Surface | Rate Constant (h−1) | R2 | Half-Life (h) |
|---|---|---|---|
| Light Control | 1.74 × 10−3 | 0.994 | 398 |
| Kaolinite Solar | 1.05 × 10−3 | 0.997 | 660 |
| Anatase Solar | 7.73 × 10−3 | 0.992 | 89.6 |
| Dark Control | 0.250 × 10−3 | 0.964 | 2770 |
| Kaolinite Dark | 1.28 × 10−3 | 0.971 | 541 |
| Anatase Dark | 4.69 × 10−3 | 0.987 | 148 |
| Surface | Rate Constant h−1 | R2 | Half-Life (h) |
|---|---|---|---|
| Light Control | 1.40 × 10−2 | 0.957 | 49.5 |
| Kaolinite Solar | 1.34 × 10−2 | 0.980 | 51.7 |
| Anatase Solar | 2.06 × 10−2 | 0.996 | 33.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellepola, N.; Rubasinghege, G. Heterogeneous Photocatalysis of Amoxicillin under Natural Conditions and High-Intensity Light: Fate, Transformation, and Mineralogical Impacts. Environments 2022, 9, 77. https://doi.org/10.3390/environments9070077
Ellepola N, Rubasinghege G. Heterogeneous Photocatalysis of Amoxicillin under Natural Conditions and High-Intensity Light: Fate, Transformation, and Mineralogical Impacts. Environments. 2022; 9(7):77. https://doi.org/10.3390/environments9070077
Chicago/Turabian StyleEllepola, Nishanthi, and Gayan Rubasinghege. 2022. "Heterogeneous Photocatalysis of Amoxicillin under Natural Conditions and High-Intensity Light: Fate, Transformation, and Mineralogical Impacts" Environments 9, no. 7: 77. https://doi.org/10.3390/environments9070077
APA StyleEllepola, N., & Rubasinghege, G. (2022). Heterogeneous Photocatalysis of Amoxicillin under Natural Conditions and High-Intensity Light: Fate, Transformation, and Mineralogical Impacts. Environments, 9(7), 77. https://doi.org/10.3390/environments9070077