Production of Microalgal Slow-Release Fertilizer by Valorizing Liquid Agricultural Digestate: Growth Experiments with Tomatoes
Abstract
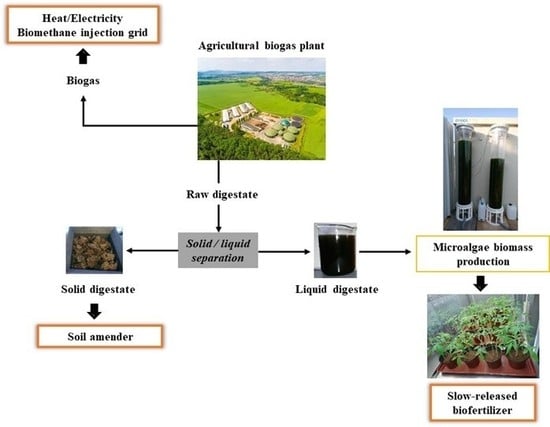
:1. Introduction
2. Materials and Methods
2.1. Microalgae, Soil and Liquid Digestate Origin
2.2. Indoor Batch Cultivation of Microalgae Testing Various Digestate Dilution
2.3. Outdoor Photo-Bioreactors Cultivation of Microalgae on the Best Dilution Ratio
2.4. Physicochemical Analysis
2.5. Agronomic Trials on Microalgae Biomass
3. Results and Discussions
3.1. Impact of Liquid-Digestate Dilution on Microalgae Growth on Batch Conditions
3.2. Cultivation of Microalgae in Outdoor Conditions
3.3. Agronomic Trials by Plant Growth and Lixiviation Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Anaerobic digestion |
| Ca | Carotenoids |
| Ch.a | Chlorophyll a |
| Ch.b | Chlorophyll b |
| CHP | Cogeneration Heat and Power system |
| OFMSW | Organic Fraction of Municipal Solid Wastes |
| TS | Total Solids |
| TSS | Total Solids Suspended |
| VS | Volatile Solids |
References
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef]
- Elalami, D.; Carrere, H.; Monlau, F.; Abdelouahdi, K.; Oukarroum, A.; Barakat, A. Pretreatment and co-digestion of wastewater sludge for biogas production: Recent research advances and trends. Renew. Sustain. Energy Rev. 2019, 114, 109287. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Costa, A.F.; Carrasco, L.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenergy 2012, 40, 181–189. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Tambone, F.; Genevini, P.; D’Imporzano, G.; Adani, F. Assessing amendment properties of digestate by studying the organic matter composition and the degree of biological stability during the anaerobic digestion of the organic fraction of msw. Bioresour. Technol. 2009, 100, 3140–3142. [Google Scholar] [CrossRef]
- Akhiar, A.; Battimelli, A.; Torrijos, M.; Carrere, H. Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion. Waste Manag. 2017, 59, 118–128. [Google Scholar] [CrossRef]
- Baral, K.R.; Jégo, G.; Amon, B.; Bol, R.; Chantigny, M.H.; Olesen, J.E.; Petersen, S.O. Greenhouse gas emissions during storage of manure and digestates: Key role of methane for prediction and mitigation. Agric. Syst. 2018, 166, 26–35. [Google Scholar] [CrossRef]
- Jiang, Y.; Pu, X.; Zheng, D.; Zhu, T.; Wang, S.; Deng, L.; Wang, W. Cultivation of lipid-producing microalgae in struvite-precipitated liquid digestate for biodiesel production. Biotechnol. Biofuels 2018, 11. [Google Scholar] [CrossRef] [Green Version]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2013, 34, 473–492. [Google Scholar] [CrossRef] [Green Version]
- Sheets, J.P.; Yang, L.; Ge, X.; Wang, Z.; Li, Y. Beyond land application: Emerging technologies for the treatment and reuse of anaerobically digested agricultural and food waste. Waste Manag. 2015, 44, 94–115. [Google Scholar] [CrossRef] [Green Version]
- Xia, A.; Murphy, J.D. Microalgal cultivation in treating liquid digestate from biogas systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Franchino, M.; Comino, E.; Bona, F.; Riggio, V.A. Growth of three microalgae strains and nutrient removal from an agro-zootechnical digestate. Chemosphere 2013, 92, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, L.; Frison, N.; Jelic, A.; Fatone, F.; Bolzonella, D.; Ballottari, M. Microalgae cultivation on anaerobic digestate of municipal wastewater, sewage sludge and agro-waste. Int. J. Mol. Sci. 2016, 17, 1692. [Google Scholar] [CrossRef]
- Delrue, F.; Álvarez-Díaz, P.; Fon-Sing, S.; Fleury, G.; Sassi, J.-F. The environmental biorefinery: Using microalgae to remediate wastewater, a win-win paradigm. Energies 2016, 9, 132. [Google Scholar] [CrossRef]
- Marazzi, F.; Sambusiti, C.; Monlau, F.; Cecere, S.E.; Scaglione, D.; Barakat, A.; Mezzanotte, V.; Ficara, E. A novel option for reducing the optical density of liquid digestate to achieve a more productive microalgal culturing. Algal Res. 2017, 24, 19–28. [Google Scholar] [CrossRef]
- Markou, G.; Monlau, F. Nutrient recycling for sustainable production of algal biofuels. Biomassbiofuels Biochem. 2019, 6, 109–133. [Google Scholar]
- Markou, G.; Wang, L.; Ye, J.; Unc, A. Using agro-industrial wastes for the cultivation of microalgae and duckweeds: Contamination risks and biomass safety concerns. Biotechnol. Adv. 2018, 36, 1238–1254. [Google Scholar] [CrossRef]
- Markou, G.; Wang, L.; Ye, J.; Unc, A. Cultivation of microalgae on anaerobically digested agro-industrial wastes and by-products. In Application of Microalgae in Wastewater Treatment; Springer Nature: Cham, Switzerland, 2020; pp. 1–29. [Google Scholar]
- Li, J.; Wang, L.; Lu, Q.; Zhou, W. Toxicity alleviation for microalgae cultivation by cationic starch addition and ammonia stripping and study on the cost assessment. Rsc Adv. 2019, 9, 38235–38245. [Google Scholar] [CrossRef] [Green Version]
- Voloshin, R.A.; Rodionova, M.V.; Zharmukhamedov, S.K.; Nejat Veziroglu, T.; Allakhverdiev, S.I. Review: Biofuel production from plant and algal biomass. Int. J. Hydrog. Energy 2016, 41, 17257–17273. [Google Scholar] [CrossRef]
- Ayre, J.M.; Moheimani, N.R.; Borowitzka, M.A. Growth of microalgae on undiluted anaerobic digestate of piggery effluent with high ammonium concentrations. Algal Res. 2017, 24, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Uysal, O.; Uysal, F.O.; Ekinci, K. Evaluation of microalgae as microbial fertilizer. Eur. J. Sustain. Dev. 2015, 4, 77–82. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- Faheed, F.A.; Abd-El Fattah, Z. Effect of chlorella vulgaris as bio-fertilizer on growth parameters and metabolic aspects of lettuce plant. J. Agri. Soc. Sci. 2008, 4, 165–169. [Google Scholar]
- Garcia-Gonzalez, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga acutodesmus dimorphus. J. Appl. Phycol. 2015, 28, 1051–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulbry, W.; Westhead, E.K.; Pizarro, C.; Sikora, L. Recycling of manure nutrients: Use of algal biomass from dairy manure treatment as a slow release fertilizer. Bioresour. Technol. 2005, 96, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Taha, T.M.; Youssef, M.A. Improvement of growth parameters of zea mays and properties of soil inoculated with two chlorella species. Rep. Opin. 2015, 7, 22–27. [Google Scholar]
- El-Sharony, T.F.; El-Gioushy, S.F.; Amin, O.A. Effect of foliar application with algae and plant extracts on growth, yield and fruit quality of fruitful mango trees cv. Fagri kalan. J. Hortic. 2015, 2. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Kumaravel, R.; Gopalsamy, J.; Sikder, M.N.A.; Sampathkumar, P. Microalgae as bio-fertilizers for rice growth and seed yield productivity. Waste Biomass Valorization 2017, 9, 793–800. [Google Scholar] [CrossRef]
- Gell, K.; van Groenigen, J.; Cayuela, M.L. Residues of bioenergy production chains as soil amendments: Immediate and temporal phytotoxicity. J. Hazard. Mater. 2011, 186, 2017–2025. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Sogn, T.A.; Dragicevic, I.; Linjordet, R.; Krogstad, T.; Eijsink, V.G.H.; Eich-Greatorex, S. Recycling of biogas digestates in plant production: Npk fertilizer value and risk of leaching. Int. J. Recycl. Org. Waste Agric. 2018, 7, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Dhup, S.; Dhawan, V. Effect of nitrogen concentration on lipid productivity and fatty acid composition of monoraphidium sp. Bioresour. Technol. 2014, 152, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Uggetti, E.; Sialve, B.; Latrille, E.; Steyer, J.-P. Anaerobic digestate as substrate for microalgae culture: The role of ammonium concentration on the microalgae productivity. Bioresour. Technol. 2014, 152, 437–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prajapati, S.K.; Kumar, P.; Malik, A.; Vijay, V.K. Bioconversion of algae to methane and subsequent utilization of digestate for algae cultivation: A closed loop bioenergy generation process. Bioresour. Technol. 2014, 158, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Masojídek, J.; Torzillo, G. Mass Cultivation of Freshwater Microalgae; Elsevier: Amsterdam, The Netherlands, 2008; pp. 2226–2235. [Google Scholar]
- Marcilhac, C.; Sialve, B.; Pourcher, A.-M.; Ziebal, C.; Bernet, N.; Béline, F. Digestate color and light intensity affect nutrient removal and competition phenomena in a microalgal-bacterial ecosystem. Water Res. 2014, 64, 278–287. [Google Scholar] [CrossRef]
- Marín, D.; Posadas, E.; Cano, P.; Pérez, V.; Blanco, S.; Lebrero, R.; Muñoz, R. Seasonal variation of biogas upgrading coupled with digestate treatment in an outdoors pilot scale algal-bacterial photobioreactor. Bioresour. Technol. 2018, 263, 58–66. [Google Scholar] [CrossRef]
- Řezanka, T.; Nedbalová, L.; Lukavský, J.; Střížek, A.; Sigler, K. Pilot cultivation of the green alga monoraphidium sp. Producing a high content of polyunsaturated fatty acids in a low-temperature environment. Algal Res. 2017, 22, 160–165. [Google Scholar]
- Barreiro-Vescovo, S.; Barbera, E.; Bertucco, A.; Sforza, E. Integration of microalgae cultivation in a biogas production process from organic municipal solid waste: From laboratory to pilot scale. ChemEngineering 2020, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.; Arbib, Z.; Álvarez-Díaz, P.D.; Garrido-Pérez, C.; Barragán, J.; Perales, J.A. Photobiotreatment model (phbt): A kinetic model for microalgae biomass growth and nutrient removal in wastewater. Environ. Technol. 2013, 34, 979–991. [Google Scholar] [CrossRef]
- Coppens, J.; Grunert, O.; Van Den Hende, S.; Vanhoutte, I.; Boon, N.; Haesaert, G.; De Gelder, L. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 2015, 28, 2367–2377. [Google Scholar] [CrossRef]
- Da Ros, C.; Libralato, G.; Ghirardini, A.V.; Radaelli, M.; Cavinato, C. Assessing the potential phytotoxicity of digestate from winery wastes. Ecotoxicol. Environ. Saf. 2018, 150, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Solé-Bundó, M.; Cucina, M.; Folch, M.; Tàpias, J.; Gigliotti, G.; Garfí, M.; Ferrer, I. Assessing the agricultural reuse of the digestate from microalgae anaerobic digestion and co-digestion with sewage sludge. Sci. Total Environ. 2017, 586, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.D.S.; Calijuri, M.L.; Assemany, P.P.; Cecon, P.R.; de Assis, I.R.; Ribeiro, V.J. Microalgae biofilm in soil: Greenhouse gas emissions, ammonia volatilization and plant growth. Sci. Total Environ. 2017, 574, 1640–1648. [Google Scholar] [CrossRef]
- Wuang, S.C.; Khin, M.C.; Chua, P.Q.D.; Luo, Y.D. Use of spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Res. 2016, 15, 59–64. [Google Scholar] [CrossRef]





| Parameters | Values | Units |
|---|---|---|
| pH (25 °C) | 8.6 | |
| Conductivity | 3640 | µS cm−1 |
| TS | 2.0 | % raw matter |
| VS | 470 | g/kg TS |
| COD | 239 | g/kg TS |
| NTK | 216 | brut g N/kg TS |
| NH4+ | 121 | g N/kg TS |
| P | 16.1 | g P2O5/kg TS |
| K | 252 | g K2O/kg TS |
| Mg | 7.1 | g MgO/kg TS |
| Ca | 33.8 | g CaO/kg TS |
| S | 17.2 | g SO3/kg TS |
| As | 2.1 | mg As/kg TS |
| Cd | 0.36 | mg Cd/kg TS |
| Cr | 5.5 | mg Cr/kg TS |
| Cu | 79.5 | mg Cu/kg TS |
| Ni | 10.0 | mg Ni/kg TS |
| Zn | 207 | mg Zn/kg TS |
| Indoor | Outdoor | |||||
|---|---|---|---|---|---|---|
| L 20 | L 30 | L 50 | B1 | B2 | B3 | |
| Specific growth, µ (d−1) | 0.03 | 0.05 | 0.13 | 0.25 | 0.14 | 0.21 |
| Biomass productivity (mg TSS L−1 d−1) | - | - | - | 43.3 | 31.7 | 39.8 |
| Cells max (*107 cell/mL) | 0.74 | 2.2 | 3.6 | 1.7 | 1.3 | 1.6 |
| Biomass yield (g TSS. L−1) | - | - | - | 0.77 | 0.57 | 0.68 |
| NH4+ (%removal capacity) | 34 | 66 | 100 | 100 | 100 | 100 |
| P-PO4−3 (%removal capacity) | 0 | 77 | 92 | 78.5 | 46.6 | 75.6 |
| Parameters | Values (± S.D) |
|---|---|
| C (% TS) | 30.62 ± 0.22 |
| H (% TS) | 4.04 ± 0.03 |
| N (% TS) | 3.27 ± 0.26 |
| S (% TS) | 0.85 ± 0.02 |
| K(% TS) | 0.55 ± 0.02 |
| P(% TS) | 0.90 ± 0.2 |
| Ca (% TS) | 0.45 ± 0.02 |
| Mg(% TS) | 0.25 ± 0.08 |
| C (% TS) | H (% TS) | N (% TS) | S (% TS) | |
|---|---|---|---|---|
| S | 38.60 ± 0.12 | 5.94 ± 0.06 | 0.95 ± 0.03 | 0.26 ± 0.01 |
| SE | 38.13 ± 0.14 | 5.88 ± 0.01 | 1.54 ± 0.03 | 0.21 ± 0.00 |
| SD | 37.76 ± 0.03 | 5.87 ± 0.03 | 1.45 ± 0.03 | 0.28 ± 0.01 |
| SM | 38.14 ± 0.14 | 5.88 ± 0.06 | 1.04 ± 0.01 | 0.33 ± 0.02 |
| Medium Growth and Pilots Used | Microalgae Species | Crop Type | Fertilizer Application | Cultivation Conditions | Enhanced Effect of Microalgae Biomass Treatment Compared to Controls | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Grow Rate | Leaf Number or Size | |||||||
| Wastewater from aquaculture system. Microalgae cultivated in outdoor raceway pond (28 m2). | Commercial Microalgal Bacterial flocs | Tomato Solanum lycopersicum cv. | (i) liquid inorganic fertilizer, | Cultivation in greenhouses: 22 °C-13 h light, 17 °C-11 h dark. Luminosity: 50 µmol m−2 s−1. Cultivated in labs (1.0 × 0.2 × 0.085 m) with an interspacing of 50 cm between plants. | (i) 3.76 g TS (ii) 3.76 g TS (iii) 4.01 g TS (iv) 3.83 g TS | (i) 40.7 cm (ii) 40.6 cm (iii) 37.8 cm (iv) 42.2 cm | [26] | |
| (ii) solid organic fertilizer, | ||||||||
| (iii) MaB-floc fertilizer, | ||||||||
| Modified Guillard’s f/2 marine medium. Microalgae cultivated in outdoor closed flat panel photobioreactors (25 m2) | Nannochloropsis oculata | |||||||
| (iv) Nannochloropsis fertilizer. | ||||||||
| Digestate dairy manure as medium culture. Algal biomass was produced from laboratory-scale algal turf scrubbers (ATS) | Algal biomass | Cucumber (Cucumis satious) | (i) 2.8 g dry alga/pot, | Cultivation in pots located in a growth chamber. Conditions: 25 °C 16 h light, 20 °C 8 h dark, 20-days growth, 600 µmol m−2 s−1. | (i) 2.98 gTS/pot; (ii) 4.35 gTS/pot; (iii) 4.16 gTS/pot; (iv) 3.18 gTS/pot; (v) 4.55 gTS/pot; (vi) 0.99 gTS/pot; | - | [43] | |
| (ii) 5.5 g dry alga/pot, | ||||||||
| (iii) 11 g dry alga per pot, | ||||||||
| (iv) 1.25 g indust. fertilizer/pot, | ||||||||
| (v) 2.5 g industrial fertilizer/pot, | ||||||||
| (vi) no fertilization. | ||||||||
| Corn (Zea mays) | (i) 10 g dry alga/pot, | (i) 6.8 gTS/pot; (ii) 10.7 gTS/pot; (iii) 7.7 gTS/pot; (iv) 8.6 gTS/pot; (v) 0.53 gTS/pot; | ||||||
| (ii) 20 g dry alga/pot, | ||||||||
| (iii) 5.5 g indust. fertilizer/ pot, | ||||||||
| (iv) 11 g indust. fertilizer/pot, | ||||||||
| (v) no fertilization. | ||||||||
| The algal cells were grown at a temperature of 25 ± 1 °C and surface light of about 2500 lux. | Chlorella vulgaris | Lettuce (lactuca sativa cv.) | (i) no fertilization, | Cultivated in pot in greenhouse under a 16 h photoperiod at 20–25 °C for 30 days. | (i) 3.2 gTS/plant; (ii) 5.5 gTS/plant; (iii) 6.5 gTS/plant; (iv) 8.3 gTS/plant; (v) 9.8 gTS/plant; | - | [24] | |
| (ii) 1/2 g/kg of soil of dry algae, | ||||||||
| (iii) 1 g/kg of soil of dry algae, | ||||||||
| (iv) 2 g/kg of soil of dry algae, | ||||||||
| (v) 3 g/kg of soil of dry algae. | ||||||||
| Fish wastewater. Batch cultivation (2 L) of the microalgae was performed using the obtained fish water in glass bottles and air-aerated with a flow rate of 10–15 mL/min at 28–30 °C. | S. platensis (LB 1928) | Arugula (Erucasativa) | (i) no fertilization, | Each pot consists of 500 g of soil. Conditions: 13.5 µmol m−2 s−1 during 21–40 days. | (i) 4.28 gTS; (ii) 10.95 gTS; (iii) 21.16 gTS; (iv) 22.74 gTS; | (i) 9.3 leaves (ii) 11 leaves (iii) 12.3 leaves (iv) 13.3 leaves | [27] | |
| (ii) Spirulina (5 g/pot), | ||||||||
| Bayam Red (Ameranthus gangeticus), | (iii) chemical fertilizer, | (i) 7.48 gTS; (ii) 9.06 gTS; (iii) 9.52 gTS; (iv) 10 gTS; | (i) 7.6 leaves (ii) 9 leaves (iii) 9.7 leaves (iv) 9.7 leaves | |||||
| (iv) Spirulina (5 g/pot) plus chemical fertilizer. | ||||||||
| BG 11 medium. Cultivated in 1 L conical flasks by continuous air bubbling under continuous illumination 3500 lux for 7 days | Chlorella oocystoides | Zea mays | (i) Without algae (Control 0%), | Black plastic pots (15 cm in diameter and 20 cm height). | (i) 0.98 gTS/plant (ii) 1.03 gTS/plant (iii) 1.33 gTS/plant (iv) 1.5 gTS/plant (v) 1.49 gTS/plant | (i) 6 leaves (ii) 6.1 leaves (iii) 6.5 leaves (iv) 6.7 leaves (v) 6.5 leaves | [47] | |
| (ii) 0.1% of algae, | ||||||||
| (iii) 1% of algae, | ||||||||
| (iv) 2.5% of algae, | ||||||||
| (v) 5% of algae. | ||||||||
| Industrial wastewater from meat processing industry. Microalgae cultivated in a high-rate algal pond (HRAP) | Consortium of algal biomass | Millet crop, Pennisetum glaucum, (BRS 1501 cultivar) | (i) no fertilization, | Outdoors Brazil; plot of 4 m2. Duration: 60 days. | (i) 2933 kgTS ha−1 (ii) 4523 kgTS ha−1 (iii) 4979 kgTS ha−1 | 4–30%. No significant difference with industrial fertilizers | [25] | |
| (ii) 120 kg ha−1 of N provided by the algal biomass, | ||||||||
| (iii) 120 kg ha−1 of N provided by conventional urea. | ||||||||
| BG11 algae culture medium. Microalgae cultivated outdoors in seven 1.22 m × 14.63 m production row panel photobioreactors | Acutodesmus dimorphus (LARB-0414) | Roma tomato (S. lycopersicum var. Roma) seeds | (i) control (no fertilizers), | 28 °C, 85% humidity, greenhouse, 14 days growth. The seedlings were grown into 28-cm pots for 8 weeks. | (i) 47 g (weight of shoot and root) (ii) 153 g (B1) (iii) 70.5 g (B2) | 53% with 50 g of biofertilizer 88% with 100 g of biofertilizer | [46] | |
| (ii) 50 g of biofertilizer applied at the time of transplant (B1), | ||||||||
| (iii) 100 g of biofertilizer applied at the time of transplant (B2). | ||||||||
| (i) control (no fertilizers), | (i) 47 g (weight of shoot and root) (ii) 547 g (B1) (iii) 564 g (B2) | 123% with 50 g of biofertilizer 192% with 100 g of biofertilizer | ||||||
| (ii) 50 g of biofertilizer applied 22 days prior to transplant (B1), | ||||||||
| (iii) 100 g of biofertilizer applied 22 days prior to transplant (B2). | ||||||||
| Agricultural liquid digestate diluted at 1:50. Microalgae cultivated in outdoor condition in 110 L photobioreactors | Monoraphidium sp. | tomatoes (Solanum Lycopersicum) | (i) no fertilizers, (ii) industrial fertilizers 170 kgN/ha, (iii) liquid digestate 170 kg N/ha, (iv) microalgae at 70 kg N/ha. | Cultivation in 0.5 L pot in growth chamber. During the trial, the environmental conditions were the following ones: day duration 16 h at 25 °C under 4600 lux (62.1 µmol m2 s−1), in 60 % relative humidity, whereas night conditions were 8 h darkness at 18 °C in 80% relative humidity. | (i) 19.4 gTS/100 plants (ii) 24.8 gTS/100 plants (iii) 25.2 gTS/100 plants (iv) 25.7 gTS/100 plants | (i) 20.8 leaves/ plant (ii) 30 leaves/ plant (iii) 29.6 leaves/ plant (iv) 27.4 leaves/ plant | This work | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez, R.; Markou, G.; Tayibi, S.; Barakat, A.; Chapsal, C.; Monlau, F. Production of Microalgal Slow-Release Fertilizer by Valorizing Liquid Agricultural Digestate: Growth Experiments with Tomatoes. Appl. Sci. 2020, 10, 3890. https://doi.org/10.3390/app10113890
Jimenez R, Markou G, Tayibi S, Barakat A, Chapsal C, Monlau F. Production of Microalgal Slow-Release Fertilizer by Valorizing Liquid Agricultural Digestate: Growth Experiments with Tomatoes. Applied Sciences. 2020; 10(11):3890. https://doi.org/10.3390/app10113890
Chicago/Turabian StyleJimenez, Raquel, Giorgos Markou, Saida Tayibi, Abdellatif Barakat, Camille Chapsal, and Florian Monlau. 2020. "Production of Microalgal Slow-Release Fertilizer by Valorizing Liquid Agricultural Digestate: Growth Experiments with Tomatoes" Applied Sciences 10, no. 11: 3890. https://doi.org/10.3390/app10113890
APA StyleJimenez, R., Markou, G., Tayibi, S., Barakat, A., Chapsal, C., & Monlau, F. (2020). Production of Microalgal Slow-Release Fertilizer by Valorizing Liquid Agricultural Digestate: Growth Experiments with Tomatoes. Applied Sciences, 10(11), 3890. https://doi.org/10.3390/app10113890