Magnesium Oxybromides MOB-318 and MOB-518: Brominated Analogues of Magnesium Oxychlorides
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
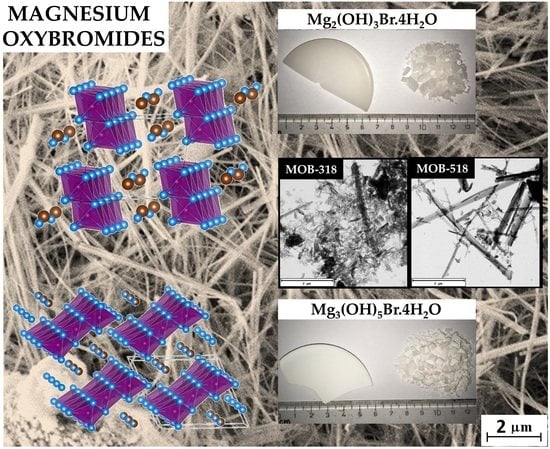
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef] [Green Version]
- Naik, T.R. Sustainability of Concrete Construction. Pract. Period. Struct. Des. Constr. 2008, 13, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Góchez, R.; Vreeland, T.; Wambaugh, J.; Kitchens, C.L. Conversion of magnesium oxychloride to chlorartinite and resulting increased water resistance. Mater. Lett. 2017, 207, 1–3. [Google Scholar] [CrossRef]
- Power, I.M.; Dipple, G.; Francis, P. Assessing the carbon sequestration potential of magnesium oxychloride cement building materials. Cem. Concr. Compos. 2017, 78, 97–107. [Google Scholar] [CrossRef]
- Harrison, J. Reactive Magnesium Oxide Cements. Patent WO 01/55049 A1, 2 August 2001. [Google Scholar]
- Walling, S.; Provis, J.L. Magnesia-Based Cements: A Journey of 150 Years, and Cements for the Future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef]
- Liška, M.; Al-Tabbaa, A. Performance of magnesia cements in pressed masonry units with natural aggregates: Production parameters optimisation. Constr. Build. Mater. 2008, 22, 1789–1797. [Google Scholar] [CrossRef]
- Biel, T.D.; Lee, H. Magnesium oxychloride cement concrete with recycled tire rubber. Transp. Res. Rec. 1996, 1561, 6–12. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Zheng, L.; Wen, J.; Wu, C.; Tan, Y. Compressive strength of fly ash magnesium oxychloride cement containing granite wastes. Constr. Build. Mater. 2013, 38, 1–7. [Google Scholar] [CrossRef]
- He, P.; Hossain, U.; Poon, C.S.; Tsang, D.C. Mechanical, durability and environmental aspects of magnesium oxychloride cement boards incorporating waste wood. J. Clean. Prod. 2019, 207, 391–399. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z. Light-weight wood–magnesium oxychloride cement composite building products made by extrusion. Constr. Build. Mater. 2012, 27, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Plekhanova, T.; Kerienė, J.; Gailius, A.; Yakovlev, G.I. Structural, physical and mechanical properties of modified wood–magnesia composite. Constr. Build. Mater. 2007, 21, 1833–1838. [Google Scholar] [CrossRef]
- Sugimoto, K.; Dinnebier, R.; Schlecht, T. Structure determination of Mg3(OH)5Cl center dot 4H2O (f5 phase) from laboratory powder diffraction data and its impact on the analysis of problematic magnesia floors. Acta Crystallogr. Sect. B Struct. Sci. 2008, 63, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Xing, P.; Gao, S. Studies on the basic compounds of magnesia cement: The thermal behaviour of magnesium oxychlorides. Thermochim. Acta 1991, 183, 349–363. [Google Scholar] [CrossRef]
- Maravelaki-Kalaitzaki, P.; Moraitou, G. Sorel’s cement mortars: Decay susceptibility and effect on pentelic marble. Cem. Concr. Res. 1999, 29, 1929–1935. [Google Scholar] [CrossRef]
- de Castellar, M.D.; Lorente, J.C.; Traveria, A.; Tura, J.M. Cracks in sorel’s cement polishing bricks as a result of magnesium oxychloride carbonatation. Cem. Concr. Res. 1996, 26, 1199–1202. [Google Scholar] [CrossRef]
- Montle, J.F.; Mayhan, K.G. The role of magnesium oxychloride as a fire-resistive material. Fire Technol. 1974, 10, 201–210. [Google Scholar] [CrossRef]
- Lojka, M.; Jankovský, O.; Jiříčková, A.; Lauermannová, A.-M.; Antončík, F.; Sedmidubský, D.; Pavlík, Z.; Pavlíková, M. Thermal stability and kinetics of formation of magnesium oxychloride phase 3Mg(OH) 2∙MgCl2∙8H2O. Materials 2020, 13, 767. [Google Scholar] [CrossRef] [Green Version]
- Góchez, R.; Wambaugh, J.; Rochner, B.; Kitchens, C.L. Kinetic study of the magnesium oxychloride cement cure reaction. J. Mater. Sci. 2017, 37, 866–7646. [Google Scholar] [CrossRef]
- Sglavo, V.M.; De Genua, F.; Conci, A.; Ceccato, R.; Cavallini, R. Influence of curing temperature on the evolution of magnesium oxychloride cement. J. Mater. Sci. 2011, 46, 6726–6733. [Google Scholar] [CrossRef]
- Sorel, S. On a new magnesium cement. C. R. Acad. Sci. 1867, 65, 102–104. [Google Scholar]
- Tasilly, E. Sels basiques de magnésium. C. R. Acad. Sci. 1897, 125, 605. [Google Scholar]
- Thompson, H.C. Fireproof Product Using Magnesium Oxychloride Cement. U.S. Patent 3,963,849, 15 June 1976. [Google Scholar]
- Li, G.; Yu, Y.; Li, J.; Wang, Y.; Liu, H. Experimental study on urban refuse/magnesium oxychloride cement compound floor tile. Cem. Concr. Res. 2003, 33, 1663–1668. [Google Scholar] [CrossRef]
- Qiao, H.; Cheng, Q.; Wang, J.; Shi, Y. The application review of magnesium oxychloride cement. J. Chem. Pharm. Res. 2014, 6, 180–185. [Google Scholar]
- Urwongse, L.; Sorrell, C.A. The system MgO-MgCl2-H2O at 23 °C. J. Am. Ceram. Soc. 1980, 63, 501–504. [Google Scholar] [CrossRef]
- Li, Z.; Chau, C. Influence of molar ratios on properties of magnesium oxychloride cement. Cem. Concr. Res. 2007, 37, 866–870. [Google Scholar] [CrossRef]
- Dorrepaal, R.; Gowen, A. Identification of Magnesium Oxychloride Cement Biomaterial Heterogeneity using Raman Chemical Mapping and NIR Hyperspectral Chemical Imaging. Sci. Rep. 2018, 8, 13034. [Google Scholar] [CrossRef] [PubMed]
- Matković, B.; Young, J.F. Microstructure of Magnesium Oxychloride Cements. Nat. Phys. Sci. 1973, 246, 79–80. [Google Scholar] [CrossRef]
- De Wolff, P.M.; Walter-Lévy, L. The crystal structure of Mg2(OH)3(Cl,Br).4H2O. Acta Crystallogr. 1953, 6, 40–44. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Shi, E.; Wang, A.; Ling, Z. MIR, VNIR, NIR, and Raman spectra of magnesium chlorides with six hydration degrees: Implication for Mars and Europa. J. Raman Spectrosc. 2019, 1–14. [Google Scholar] [CrossRef]
- Sugimoto, K.; Dinnebier, R.E.; Hanson, J.C. Structures of three dehydration products of bischofite from in situ synchrotron powder diffraction data (MgCl2 n H2O; n = 1, 2, 4). Acta Crystallogr. Sect. B Struct. Sci. 2007, 63, 235–242. [Google Scholar] [CrossRef]
- Jiříčková, A.; Lojka, M.; Lauermannová, A.-M.; Antončík, F.; Sedmidubský, D.; Pavlíková, M.; Záleská, M.; Pavlik, Z.; Jankovský, O. Synthesis, structure, and thermal stability of magnesium oxychloride 5Mg(OH)2∙MgCl2∙8H2O. Appl. Sci. 2020, 10, 1683. [Google Scholar] [CrossRef] [Green Version]
- Jankovský, O.; Lojka, M.; Lauermannová, A.-M.; Antončík, F.; Pavlíková, M.; Pavlík, Z.; Sedmidubský, D. Carbon Dioxide Uptake by MOC-Based Materials. Appl. Sci. 2020, 10, 2254. [Google Scholar] [CrossRef] [Green Version]









| Element | Content in MgBr2·6H2O (wt%) | Content in MgO (wt%) |
|---|---|---|
| Mg | 11.44 | 99.19 |
| P | 0.01 | - |
| Cl | 0.10 | 0.38 |
| Br | 88.45 | 0.04 |
| Ca | - | 0.16 |
| Al | - | 0.13 |
| S | - | 0.10 |
| Wavenumbers (cm−1) | Assignment |
|---|---|
| 3648, 3623 | Stretching modes (ν) of O-H in Mg(OH)2 |
| 3370 | Stretching modes (ν) of H-O-H in H2O |
| 2050 | Bending (δ) and rocking (ρ) vibrations of H-O-H in H2O |
| 1608 | Bending (δ) vibration of H-O-H in MgBr2·8H2O |
| 1523, 1445 | Stretching modes (ν) of Mg-O MgBr2·8H2O |
| 853 | Stretching vibration (ν) of Mg-O cubic structure |
| 574 | Deformation (δ) and stretching (ν) vibrations of Mg-Br |
| 420 | Vibrational modes of the lattice showing the Mg-O/Mg2+, O/O-Mg-O/O-Mg2+-O bonds |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauermannová, A.-M.; Lojka, M.; Antončík, F.; Sedmidubský, D.; Pavlíková, M.; Pavlík, Z.; Jankovský, O. Magnesium Oxybromides MOB-318 and MOB-518: Brominated Analogues of Magnesium Oxychlorides. Appl. Sci. 2020, 10, 4032. https://doi.org/10.3390/app10114032
Lauermannová A-M, Lojka M, Antončík F, Sedmidubský D, Pavlíková M, Pavlík Z, Jankovský O. Magnesium Oxybromides MOB-318 and MOB-518: Brominated Analogues of Magnesium Oxychlorides. Applied Sciences. 2020; 10(11):4032. https://doi.org/10.3390/app10114032
Chicago/Turabian StyleLauermannová, Anna-Marie, Michal Lojka, Filip Antončík, David Sedmidubský, Milena Pavlíková, Zbyšek Pavlík, and Ondřej Jankovský. 2020. "Magnesium Oxybromides MOB-318 and MOB-518: Brominated Analogues of Magnesium Oxychlorides" Applied Sciences 10, no. 11: 4032. https://doi.org/10.3390/app10114032
APA StyleLauermannová, A. -M., Lojka, M., Antončík, F., Sedmidubský, D., Pavlíková, M., Pavlík, Z., & Jankovský, O. (2020). Magnesium Oxybromides MOB-318 and MOB-518: Brominated Analogues of Magnesium Oxychlorides. Applied Sciences, 10(11), 4032. https://doi.org/10.3390/app10114032