Characterization of Bioactive Ligands with Antioxidant Properties of Kiwifruit and Persimmon Cultivars Using In Vitro and in Silico Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Determination of Firmness, Total Soluble Solids (TSS), pH, Total Acidity (TA), and Dry Matter
2.4. Sample Extraction
2.5. Total Contents (T) of Phenolics (TPs), Flavonoids (TFAs), Flavanols (TFLs), Tannins (TNs), Vitamin C (VC) and Main Secondary Metabolites
2.6. Antioxidant Capacities
2.7. Fluorometric Measurements
2.8. Protein and Ligand Preparation for Molecular Docking Studies
2.9. Statistical Analysis
3. Results and Discussion
3.1. Determination of Harvest Parameters and Physicochemical Properties of Kiwifruit and Persimmon
3.2. Biologically Active Compounds and Secondary Metabolites in Investigated Fruits
3.3. Antioxidant Capacities
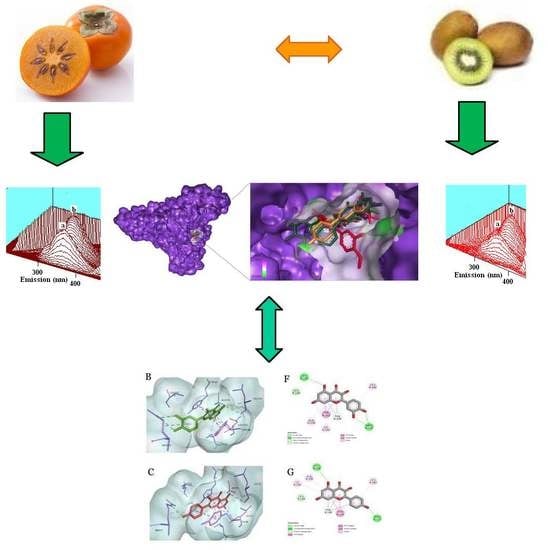
3.4. Binding Properties of Bioactive Compounds of Investigated Fruits with Serum Protein Measured by Fluorescence
3.5. Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leontowicz, H.; Leontowicz, M.; Latocha, P.; Jesion, I.; Park, Y.-S.; Katrich, E.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Bioactivity and nutritional properties of hardy kiwifruit Actinidia arguta in comparison with Actinidia deliciosa ‘Hayward’ and Actinidia eriantha ‘Bidan’. Food Chem. 2016, 196, 281–291. [Google Scholar] [CrossRef]
- Hamid Abdul, N.A.; Mediani, A.; Maulidiani, M.; Abas, F.; Park, Y.S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Gorinstein, S. Characterization of metabolites in different kiwifruit varieties by NMR and fluorescence spectroscopy. J. Pharm. Biomed. Anal. 2017, 138, 80–91. [Google Scholar] [CrossRef]
- Han, N.; Park, H.; Kim, C.W.; Kim, M.S.; Lee, U. Physicochemical quality of hardy kiwifruit (Actinidia arguta L. cv. Cheongsan) during ripening is influenced by harvest maturity. Forest Sci. Technol. 2019, 154, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Maulidiani, M.; Mediani, A.; Abas, F.; Park, Y.S.; Park, Y.K.; Kim, Y.M.; Gorinstein, S. 1H NMR and antioxidant profiles of polar and non-polar extracts of persimmon (Diospyros kaki L.) -Metabolomics study based on cultivars and origins. Talanta 2018, 184, 277–286. [Google Scholar] [CrossRef]
- Maulidiani, M.; Abdul-Hamid, N.A.; Abas, F.; Park, Y.S.; Park, Y.-K.; Kim, Y.M.; Gorinstein, S. Detection of bioactive compounds in persimmon (Diospyros kaki) using UPLC-ESI-Orbitrap-MS/MS and fluorescence analyses. Microchem. J. 2019, 149, 103978. [Google Scholar] [CrossRef]
- Alim, A.; Li, T.; Nisar, T.; Ren, D.; Liu, Y.; Yang, X. Consumption of two whole kiwifruit (Actinidia chinensis) per day improves lipid homeostasis, fatty acid metabolism and gut microbiota in healthy rats. Int. J. Biol. Macromol. 2020, 156, 186–195. [Google Scholar] [CrossRef]
- Li, D.; Zhu, F. Physicochemical, functional and nutritional properties of kiwifruit flour. Food Hydrocoll. 2019, 92, 250–258. [Google Scholar] [CrossRef]
- Dario, D.; Mellano, M.G.; Riondato, I.; De Biaggi, M.; Gamba, G.; Beccaro, G.L.; Andriamaniraka, H. Traditional and unconventional dried fruit snacks as a source of health-promoting compounds. Antioxidants 2019, 8, 396. [Google Scholar]
- Guo, J.; Yuan, Y.; Dou, P.; Yue, T. Multivariate statistical analysis of the polyphenolic constituents in kiwifruit juices to trace fruit varieties and geographical origins. Food Chem. 2017, 232, 552–559. [Google Scholar] [CrossRef]
- Park, Y.S.; Ham, K.S.; Park, Y.K.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Katrich, E.; Gorinstein, S. The effects of treatment on quality parameters of smoothie-type ‘Hayward’ kiwi fruit beverages. Food Control 2016, 70, 221–228. [Google Scholar] [CrossRef]
- Ma, T.; Lan, T.; Geng, T.; Ju, Y.; Cheng, G.; Que, Z.; Gao, G.; Fang, Y.; Sun, X. Nutritional properties and biological activities of kiwifruit (Actinidia) and kiwifruit products under simulated gastrointestinal in vitro digestion. Food Nutr. Res. 2019, 63, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C. Kiwifruit and cancer: An overview of biological evidence. Nutr. Cancer 2020, 72, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Sultan, M.T.; Aziz, M.; Naz, A.; Ahmed, W.; Kumar, N.; Imran, M. Persimmon (Diospyros Kaki) fruit: Hidden phytochemicals and health claims. Excli J. 2015, 14, 42–561. [Google Scholar]
- Yaqub, S.; Farooq, U.; Shafi, A.; Akram, K.; Murtaza, M.A.; Kausar, T.; Siddique, F. Chemistry and functionality of bioactive compounds present in persimmon. J. Chem. 2016, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Lu, W.Q.; Zhou, X.M. Phenolic compounds and in vitro antibacterial and antioxidant activities of three tropic fruits: Persimmon, guava, and sweetsop. Biomed Res. Int. 2016, 2016, 9. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Wang, R.F.; Khalifa, I.; Li, C.M. Understanding toward the biophysical interaction of polymeric proanthocyanidins (persimmon condensed tannins) with biomembranes: Relevance for biological effects. J. Agric. Food Chem. 2019, 67, 11044–11052. [Google Scholar] [CrossRef]
- Park, Y.S.; Jung, S.T.; Kang, S.G.; Delgado-Licon, E.; Martinez-Ayala, A.M.; Tapia, M.S.; Martin-Belloso, O.; Trakhtenberg, S.; Gorinstein, S. Drying of persimmons (Diospyros kaki L.) and the following changes in the studied bioactive compounds and the total radical scavenging activities. LWT-Food Sci. Technol. 2006, 39, 748–755. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Park, S.J.; Ahn, G.H.; Garcia, C.V.; Choi, S.J.; Kim, J.T. Preconditioning heat and humidity treatment for decreasing storage defects in ‘Fuyu’ persimmon. N. Z. J. Crop Hortic. Sci. 2018, 46, 212–223. [Google Scholar] [CrossRef]
- Lee, I.; Im, S.B.; Jin, C.R.; Heo, H.J.; Cho, Y.S.; Baik, M.Y.; Kim, D.O. Effect of maturity stage at harvest on antioxidant capacity and total phenolics in kiwifruits (Actinidia spp.) grown in Korea. Hortic. Environ. Biotechnol. 2015, 56, 841–848. [Google Scholar] [CrossRef]
- Chen, X.N.; Fan, J.F.; Yue, X.; Wu, X.R.; Li, L.T. Radical scavenging activity and phenolic compounds in persimmon (Diospyros kaki L. Cv. Mopan). J. Food Sci. 2008, 73, 24–28. [Google Scholar] [CrossRef]
- Greenfield, H.; Southgate, D.A.T. Food Composition Data: Production, Management and Use, 2nd ed.; FAO: Rome, Italy, 2003; Chapter 5, pp. 74–76, Chapter 9, pp. 165–168. [Google Scholar]
- Park, Y.S.; Im, M.H.; Choi, J.H.; Lee, H.C.; Ham, K.S.; Kang, S.G.; Park, Y.K.; Suhaj, M.; Namiesnik, J.; Gorinstein, S. Effect of long-term cold storage on physicochemical attributes and bioactive components of kiwifruit cultivars. Cyta-J. Food 2014, 12, 360–368. [Google Scholar] [CrossRef]
- Altuntas, E.; Cangi, R.; Kay, C. Physical and chemical properties of persimmon fruit. Int. Agrophys. 2011, 25, 89–92. [Google Scholar]
- Godlewska, K.; Michalak, I.; Tuhy, A.; Chojnacka, K. The influence of pH of extracting water on the composition of seaweed extracts and their beneficial properties on Lepidium sativum. Biomed Res. Int. 2017, 2017, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food Sci. F. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Feucht, W.; Polster, J. Nuclei of plants as a sink for flavanols. Z. Naturforsch. C J. Biosci. 2001, 56, 479–481. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Ozyurek, M.; Guclu, K.; Bektasoglu, B.; Apak, R. Spectrophotometric determination of ascorbic acid by the modified CUPRAC method with extractive separation of flavonoids–La (III) complexes. Anal. Chim. Acta 2007, 588, 88–95. [Google Scholar] [CrossRef]
- Park, Y.S.; Namiesnik, J.; Vearasilp, K.; Leontowicz, H.; Leontowicz, M.; Barasch, D.; Nemirovski, A.; Trakhtenberg, S.; Gorinstein, S. Bioactive compounds and the antioxidant capacity in new kiwi fruit cultivars. Food Chem. 2014, 165, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Brand-WIlliams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Norgan, A.P.; Coffman, P.K.; Kocher, J.P.A.; Katzmann, D.J.; Sosa, C.P. Multilevel parallelization of AutoDock 4.2. J. Cheminformatics 2011, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Systemes, D. BIOVIA, Discovery Studio Modeling Environment. Release 4.5; Dassault Systemes: San Diego, CA, USA, 2015. [Google Scholar]
- Kim, Y.M.; Park, Y.S.; Park, Y.K.; Ham, K.S.; Kang, S.G.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Phytochemical analysis of two main varieties of persimmon and kiwifruit and their antioxidative and quenching capacities. Eur. Food Res. Technol. 2020, 246, 1259–1268. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, M.; Shi, Y.; Yan, H.; Han, J.; Liu, L. Effect of (-)-epicatechin-3-gallate and (-)-epigallocatechin-3-gallate on the binding of tegafur to human serum albumin as determined by spectroscopy, isothermal titration calorimetry, and molecular docking. J. Biomol. Struct. Dyn. 2019, 37, 2776–2788. [Google Scholar] [CrossRef]
- Kim, C.W.; Oh, S.I.; Kim, M.J.; Park, Y. Optimal harvest time by the seasonal fruit quality and ripening characteristics of hardy kiwifruit in Korea. J. Korean Soc. For. Sci. 2014, 103, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Krupa, T.; Latocha, P.; Liwinska, A. Changes of physicochemical quality, phenolics and vitamin C content in hardy kiwifruit (Actinidia arguta and its hybrid) during storage. Sci. Hortic. 2011, 130, 410–417. [Google Scholar] [CrossRef]
- Mittelstadt, G.; Negron, L.; Schofield, L.R.; Marsh, K.; Parker, E.J. Biochemical and structural characterisation of dehydroquinate synthase from the New Zealand kiwifruit Actinidia chinensis. Arch. Biochem. Biophys. 2013, 537, 185–191. [Google Scholar] [CrossRef]
- Denoya, G.I.; Pataro, G.; Ferrari, G. Effects of postharvest pulsed light treatments on the quality and antioxidant properties of persimmons during storage. Postharvest Biol. Technol. 2020, 160, 111055. [Google Scholar] [CrossRef]
- Perez-Burillo, S.; Oliveras, M.J.; Quesada, J.; Rufian-Henares, J.A.; Pastoriza, S. Relationship between composition and bioactivity of persimmon and kiwifruit. Food Res.Intern. 2018, 105, 461–472. [Google Scholar] [CrossRef]
- Matheus, J.R.V.; de Andrade, C.J.; Miyahira, R.F.; Fai, A.E.C. Persimmon (Diospyros Kaki L.): Chemical properties, bioactive compounds and potential use in the development of new products-A Review. Food Rev. Int. 2020. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, R.M.; Zhang, Y.F.; Yang, Y.H.; Sun, X.H.; Zhang, Q.H.; Yang, N. Biotransformation of phenolics and metabolites and the change in antioxidant activity in kiwifruit induced by Lactobacillus plantarum fermentation. J. Sci. Food Agric. 2020, 100, 3283–3290. [Google Scholar] [CrossRef]
- Khatun, S.; Yasmeen, S.; Kumar, A.; Subbarao, N. Calorimetric, spectroscopic and molecular modelling insight into the interaction of gallic acid with bovine serum albumin. J. Chem. Thermodyn. 2018, 122, 85–94. [Google Scholar] [CrossRef]
- Zhang, L.L.; Liu, Y.C.; Wang, Y.M. Interaction between an (-)-epigallocatechin-3-gallate-copper complex and bovine serum albumin: Fluorescence, circular dichroism, HPLC, and docking studies. Food Chem. 2019, 301, UNSP 125294. [Google Scholar] [CrossRef]
- Zunszain, P.A.; Ghuman, J.; Komatsu, T.; Tsuchida, E.; Curry, S. Crystal structural analysis of human serum albumin complexed with hemin and fatty acid. BMC Struct. Biol. 2003, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Fasano, M.; Curry, S.; Terreno, E.; Galliano, M.; Fanali, G.; Narciso, P.; Notari, S.; Ascenzi, P. The extraordinary ligand binding properties of human serum albumin. IUBMB Life 2005, 57, 787–796. [Google Scholar] [CrossRef]
- Dizdarevic, L.L.; Biswas, D.; Uddin, M.D.M.; Jørgenesen, A.; Falch, E.; Bastani, N.E.; Duttaroy, A.K. Inhibitory effects of kiwifruit extract on human platelet aggregation and plasma angiotensin-converting enzyme activity. Platelets 2014, 25, 567–575. [Google Scholar] [CrossRef]
- Zandi, K.; Teoh, B.-T.; Sam, S.-S.; Wong, P.-F.; Mustafa, M.R.; AbuBakar, S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011, 8, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, F.-J.; Lin, C.-W.; Lai, C.-C.; Lan, Y.-C.; Lai, C.-H.; Hun, C.-H.; Hsueh, K.-C.; Lin, T.-H.; Chang, L.W.; Sheu, J.J.-C.; et al. Kaempferol inhibits enterovirus 71 replication and internal ribosome entry site (IRES) activity through FUBP and HNRP proteins. Food Chem. 2011, 128, 312–322. [Google Scholar] [CrossRef] [PubMed]
- González-Búrquez, M.D.J.; González-Díaz, F.R.; García-Tovar, C.G.; Carrillo-Miranda, L.; Soto-Zárate, C.I.; Canales-Martínez, M.M.; Penieres-Carrillo, J.G.; Crúz-Sánchez, T.A.; Fonseca-Coronado, S. Comparison between in vitro antiviral effect of Mexican propolis and three commercial flavonoids against canine distemper virus. Evid. Based Complement. Alternat. Med. 2018, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]




| Extracts of Fruits | ADH | AEB | AAC | DKF |
|---|---|---|---|---|
| TPs, mg GAE | 6.27 ± 0.43 c | 32.37 ± 1.34 a | 16.94 ± 0.81 b | 4.31 ± 0.21 c |
| TFAs, mg CE | 1.89 ± 0.22 b | 2.18 ± 0.14 b | 4.46 ± 0.31 a | 1.11 ± 0.18 c |
| TFLs, µg CE | 130 ± 6.17 b | 30 ± 2.21 c | 1790 ± 16.21 a | 83 ± 3.54 b |
| TNs, mg CE | 2.21 ± 0.12 b | 0.57 ± 0.11 c | 4.18 ± 0.31 a | 3.94 ± 0.21 a |
| VC, mg AA | 4.07 ± 0.25 b,c | 36.51 ± 1.65 a | 6.69 ± 0.43 b | 2.31 ± 0.23 c |
| ABTS, μM TE | 20.42 ± 1.17 b,c | 86.14 ± 3.23 a | 49.42 ± 2.34 b | 16.48 ± 0.86 c |
| FRAP, μMTE | 10.28 ± 0.84 c | 42.97 ± 2.45 a | 24.47 ± 1.67 b | 9.03 ± 0.34 c |
| CUPRAC, μMTE | 25.98 ± 0.96 c | 103.81 ± 5.43 a | 65.21 ± 3.56 b | 19.61 ± 0.93 c |
| DPPH, μMTE | 12.45 ± 1.12 c | 51.67 ± 2.34 a | 30.08 ± 1.98 b | 9.58 ± 0.76 c |
| FI of peak a, A.U. | 465.5 ± 7.8 a | 279.2 ± 4.3 b | 320.1 ± 4.4 b | 490.2 ± 5.4 a |
| FI of peak b, A.U. | 822.6 ± 10.6 a | 753.1 ± 7.4 b | 783.5 ± 8.7 b | 827.7 ± 6.5 a |
| BP, peak a% | 18.2 ± 1.1 c | 50.9 ± 2.34 a | 43.7 ± 1.2 b | 13.8 ± 0.6 d |
| BP, peak b% | 3.1 ± 0.2 c | 11.3 ± 1.1 a | 7.7 ± 0.6 b | 2.5 ± 0.1 c |
| Samples | λ em. nm | FI A.U. | BP (%) |
|---|---|---|---|
| HSA | 355 | 953.2 ± 6.9 a | - |
| HSA + ADH | 350 | 830.1 ± 4.8 a,b | 12.0 ± 1.4 c,d |
| HSA + GA | 354 | 817.8 ± 3.7 b | 14.2 ± 1.5 c |
| HSA + Epica | 335 | 729.4 ± 3.1 b,c | 23.5 ± 1.7 b |
| HSA + AAC | 355 | 676.3 ± 2.8 c | 29.1 ± 1.3 a,b |
| HSA + AEB | 357 | 626.6 ± 2.6 c | 34.3 ± 2.8 a |
| HSA + DKF | 355 | 865.4 ± 4.4 a,b | 9.2 ± 0.9 d |
| S. No | PubChem ID | Compounds | HSA + Kiwifruit | |||
|---|---|---|---|---|---|---|
| BE | DS | VDW | HB | |||
| 1 | 5280863 | Kaempferol | −106.3 | −138.14 | −102.8 | −3.4 |
| 2 | 5280805 | Rutin | −78.9 | −82.84 | −61.9 | −16.9 |
| 3 | 689043 | Caffeic acid | −75.0 | −99.36 | −71.5 | −3.5 |
| 4 | 6508 | Quinic acid | −69.4 | −95.18 | −63.0 | −6.3 |
| 5 | 289 | Catechol | −50.8 | −61.67 | −47.3 | −3.4 |
| 6 | 10742 | Syringic acid | −0.5 | −107.18 | −77.2 | −3.2 |
| 7 | 10621 | Hesperidin | −140.3 | −168.71 | −126.1 | −14.1 |
| 8 | 5280343 | Quercetin | −116.8 | −163 | −106.2 | −10.5 |
| 9 | 72 | Protocatechuic acid | −69.0 | −90.03 | −66.2 | −2.7 |
| S. No | PubChem ID | Compounds | HSA + Persimmon‘Fuyu’ | |||
|---|---|---|---|---|---|---|
| BE | DS | VDW | HB | |||
| 1 | 370 | Gallic acid | −72 | −95.4 | −68.7 | −3.2 |
| 2 | 72276 | Epicatechin | −103.2 | −137.4 | −92.6 | −10.4 |
| 3 | 5280863 | Kaempferol | −106.3 | −138.14 | −102.8 | −3.4 |
| 4 | 8468 | Vanillic acid | −71.6 | −95.25 | −68.1 | −3.4 |
| 5 | 689043 | Caffeic acid | −75.0 | −99.36 | −71.5 | −3.5 |
| 6 | 5280343 | Quercetin | −116.8 | −163 | −106.2 | −10.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.M.; Park, Y.S.; Park, Y.-K.; Ham, K.-S.; Kang, S.-G.; Shafreen, R.M.B.; Lakshmi, S.A.; Gorinstein, S. Characterization of Bioactive Ligands with Antioxidant Properties of Kiwifruit and Persimmon Cultivars Using In Vitro and in Silico Studies. Appl. Sci. 2020, 10, 4218. https://doi.org/10.3390/app10124218
Kim YM, Park YS, Park Y-K, Ham K-S, Kang S-G, Shafreen RMB, Lakshmi SA, Gorinstein S. Characterization of Bioactive Ligands with Antioxidant Properties of Kiwifruit and Persimmon Cultivars Using In Vitro and in Silico Studies. Applied Sciences. 2020; 10(12):4218. https://doi.org/10.3390/app10124218
Chicago/Turabian StyleKim, Young Mo, Yong Seo Park, Yang-Kyun Park, Kyung-Sik Ham, Seong-Gook Kang, Raja Mohamed Beema Shafreen, Selvaraj Alagu Lakshmi, and Shela Gorinstein. 2020. "Characterization of Bioactive Ligands with Antioxidant Properties of Kiwifruit and Persimmon Cultivars Using In Vitro and in Silico Studies" Applied Sciences 10, no. 12: 4218. https://doi.org/10.3390/app10124218
APA StyleKim, Y. M., Park, Y. S., Park, Y. -K., Ham, K. -S., Kang, S. -G., Shafreen, R. M. B., Lakshmi, S. A., & Gorinstein, S. (2020). Characterization of Bioactive Ligands with Antioxidant Properties of Kiwifruit and Persimmon Cultivars Using In Vitro and in Silico Studies. Applied Sciences, 10(12), 4218. https://doi.org/10.3390/app10124218