Impact of Microwave Plasma Torch on the Yeast Candida glabrata
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
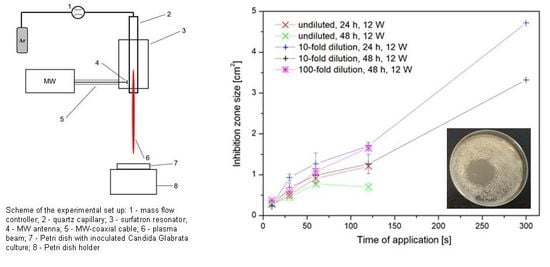
2.1. Microwave Plasma Jet Set Up
2.2. Preparation of Candida Glabrata Samples
2.3. Application of the Discharge
2.4. Statistical Analysis
3. Results and Discussion
- (a)
- Plasma exposure time;
- (b)
- Applied microwave power;
- (c)
- Initial cell concentration.
3.1. Plasma Exposure Time Impact
3.2. Applied Power Impact
3.3. Initial Cell Concentration Impact
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grzegorzewski, F. Influence of Non-Thermal Plasma Species on the Structure and Functionality of Isolated and Plantbased 1,4-Benzopyrone Derivatives and Phenolic Acids. Ph.D. Thesis, Technische Universität Berlin, Berlin, German, 2011. [Google Scholar]
- Soloshenko, I.A.; Tsiolko, V.V.; Pogulay, S.S.; Kalyuzhnaya, A.G.; Bazhenov, V.Y.; Shchedrin, A.I. Effect of Water Adding on Kinetics Of Barrier Discharge in Air. Plasma Sources Sci. Technol. 2009, 18, 045019. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Fridman, G.; Dridman, A. Physical and Biological Mechanisms of Direct Plasma Interaction with Living Tissue. N. J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Misra, N.; Tiwari, B.K.; Raghavarao, K.S.M.S.; Cullen, P.J. Nonthermal Plasma Inactivation of Food-Borne Pathogens. Food Eng. Rev. 2011, 3, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Fröhling, A.; Durek, J.; Schnabel, U.; Ehlbeck, J.; Bolling, J.; Schlüter, O. Indirect Plasma Treatment of Fresh Pork: Decontamination Efficiency and Effects on Quality Attributes. Innov. Food Sci. Emerg. Technol. 2012, 16, 381–390. [Google Scholar] [CrossRef]
- Hertwig, C.; Reineke, K.; Ehlbeck, J.; Erdoğdu, B.; Rauh, C.; Schlüter, O. Impact of Remote Plasma Treatment on Natural Microbial Load and Quality Parameters of Selected Herbs and Spices. J. Food Eng. 2015, 167, 12–17. [Google Scholar] [CrossRef]
- Ehlbeck, J.; Schnabel, U.; Andrasch, M.; Stachowiak, J.; Stolz, N.; Fröhling, A.; Schlüter, O.; Weltmann, K.-D. Plasma Treatment of Food. Contrib. Plasma Phys. 2015, 55, 753–757. [Google Scholar] [CrossRef]
- Moritz, M.; Wiacek, C.; Koethe, M.; Braun, P.G. Atmospheric Pressure Plasma Jet Treatment of Salmonella Enteritidis Inoculated Eggshells. Int. J. Food Microbiol. 2017, 245, 22–28. [Google Scholar] [CrossRef]
- Won, M.Y.; Lee, S.J.; Min, S.C. Mandarin Preservation by Microwave-Powered Cold Plasma Treatment. Innov. Food Sci. Emerg. Technol. 2017, 39, 25–32. [Google Scholar] [CrossRef]
- Lukeš, P.; Locke, B.R.; Brisset, J.L. Aqueous-phase chemistry of electrical discharge plasma in water and in gas–liquid environments. In Plasma Chemistry and Catalysis in Gases and Liquids; Parvulescu, V.I., Magureanu, M., Lukeš, P., Eds.; Wiley: Weinheim, Germany, 2012; pp. 243–308. [Google Scholar]
- Ono, R.; Oda, T. Ozone Production Process in Pulsed Positive Dielectric Barrier Discharge. J. Phys. D Appl. Phys. 2007, 40, 176–184. [Google Scholar] [CrossRef]
- Koutchma, T. UV Light for Processing Foods. Ozone Sci. Eng. 2008, 30, 93–98. [Google Scholar] [CrossRef]
- Choudhary, R.; Bandla, S. Ultraviolet Pasteurization for Food Industry. Int. J. Food Sci. Nutr. Eng. 2012, 2, 12–15. [Google Scholar] [CrossRef]
- Sosnin, E.A.; Stoffels, E.; Erofeev, M.V.; Kieft, I.E.; Kunts, S.E. The Effects of UV Irradiation and Gas Plasma Treatment on Living Mammalian Cells and Bacteria: A Comparative Approach. IEEE Trans. Plasma Sci. 2004, 32, 1544–1550. [Google Scholar] [CrossRef]
- Lu, P.; Cullen, P.J.; Ostrikov, K. Chapter 4—Atmospheric pressure nonthermal plasma sources. In Cold Plasma in Food and Agriculture: Fundamentals and Applications; Misra, N.N., Schluter, O., Cullen, P.J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 83–116. [Google Scholar]
- Lee, T.; Puligundla, P.; Mok, C. Corona Discharge Plasma Jet Inactivates Food-Borne Pathogens Adsorbed Onto Packaging Material Surfaces. Packag. Technol. Sci. 2017, 30, 681–690. [Google Scholar] [CrossRef]
- Jayasena, D.; Kim, H.J.; Cheorun, J. Dielectric barrier discharge: Meat treatment. In Encyclopedia of Plasma Technology, 1st ed.; Shohet, J.L., Ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Zhang, B.; Chen, L.; Lin, L.X.; Zhang, H. Understanding the Multi-Scale Structure and Functional Properties of Starch Modulated by Glow-Plasma: A Structure-Functionality Relationship. Food Hydrocoll. 2015, 50, 228–236. [Google Scholar] [CrossRef]
- Stoffels, E.; Flikweert, A.J.; Stoffels, W.W.; Kroesen, G.M.W. Plasma Needle: A Non-Destructive Atmospheric Plasma Source for Fine Surface Treatment of (Bio)Materials. Plasma Sources Sci. Technol. 2002, 11, 383–388. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Yuan, Y.; Fan, Y.; Guo, K.; Yue, T. Inactivation of Yeast in Apple Juice Using Gas-Phase Surface Discharge Plasma Treatment with a Spray Reactor. LWT 2018, 97, 530–536. [Google Scholar] [CrossRef]
- Marinova, P.; Benova, E.; Todorova, Y.; Topalova, Y.; Yotinov, I.; Atanasova, M.; Krcma, F. Surface-Wave-Sustained Plasma Torch for Water Treatment. J. Phys. Conf. Ser. 2018, 982, 530–536. [Google Scholar] [CrossRef]
- Stulic, V.; Vukusic, T.; Butorac, A.; Popovic, D.; Herceg, Z. Proteomic Analysis of Saccharomyces Cerevisiae Response to Plasmatreatment. Int. J. Food Microbiol. 2019, 292, 171–183. [Google Scholar] [CrossRef]
- Čtvrtečková, L.; Píchová, A.; Scholtz, V.; Khun, J.; Julák, J. Non-Thermal Plasma-Induced Apoptosis in Yeast Saccharomyces Cerevisiae. Contrib. Plasma Phys. 2019, 59, e201800064. [Google Scholar] [CrossRef]
- Polčič, P.; Pakosová, L.; Chovančíková, P.; Machala, Z. Reactive Cold Plasma Particles Generate Oxidative Stress in Yeast but do not Trigger Apoptosis. Can. J. Microbiol. 2018, 64, 367–375. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.R.; Zhu, X.; Yuan, Y.H.; Gao, Z.P.; Yue, T.L. Application of Electrical Discharge Plasma on the Inactivation of Zygosaccharomyces rouxii in Apple Juice. LWT-Food Sci. Technol. 2020, 121, 108974. [Google Scholar] [CrossRef]
- Tarabová, B.; Žilková, A.; Machala, Z. Cold Air Plasma Pasteurisation OF Fresch Apple Juice. In Proceedings of the HAKONE XV, Brno, Czech Republic, 11–16 September 2016; pp. 453–456. [Google Scholar]
- Starek, A.; Pawlat, J.; Chudzik, B.; Kwiatkowski, M.; Terebun, P.; Sagan, A.; Andrejko, D. Evaluation of Selected Microbial And Physicochemical Parameters of Fresh Tomato Juice after Cold Atmospheric Pressure Plasma Treatment during Refrigerated Storage. Sci. Rep. 2019, 9, 8407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.B.; Zhu, Y.P.; Cui, D.J.; Du, M.R.; Wang, J.Q.; Ma, R.N.; Jiao, Z. Evaluating the Roles of OH Radicals, H2O2, ORP and pH in the Inactivation of Yeast Cells on a Tissue Model By Surface Micro-Discharge Plasma. J. Phys. D Appl. Phys. 2019, 52, 395201. [Google Scholar] [CrossRef]
- Du, M.R.; Xu, H.B.; Zhu, Y.P.; Ma, R.N.; Jiao, Z. A Comparative Study of the Major Antimicrobial Agents against the Yeast Cells on the Tissue Model by Helium and Air Surface Micro-Discharge Plasma. AIP Adv. 2020, 10, 025036. [Google Scholar] [CrossRef] [Green Version]
- Benova, E.; Atanasova, M.; Bogdanov, T.; Marinova, P.; Krčma, F.; Mazánková, V.; Dostál, L. Microwave Plasma Torch at a Water Surface. Plasma Med. 2016, 6, 59–65. [Google Scholar] [CrossRef]
- Benova, E.; Marinova, P.; Atanasova, M.; Petrova, T. Surface-Wave-Sustained Argon Plasma Kinetics from Intermediate to Atmospheric Pressure. J. Phys. D Appl. Phys. 2018, 51, 474004. [Google Scholar] [CrossRef]
- Bogdanov, T.; Benova, E. Theoretical Parametric Investigation of Plasma Sustained by Traveling Electromagnetic Wave in Coaxial Configuration. Vacuum 2018, 155, 280–291. [Google Scholar] [CrossRef]
- Krčma, F.; Tsonev, I.; Smejkalová, K.; Truchlá, D.; Kozáková, Z.; Zhekova, M.; Marinova, P.; Bogdanov, T.; Benova, E. Microwave Micro Torch Generated in Argon Based Mixtures for Biomedical Applications. J. Phys. D Appl. Phys. 2018, 51, 414001. [Google Scholar] [CrossRef]
- Bogdanov, T.; Tsonev, I.; Marinova, P.; Benova, E.; Rusanov, K.; Rusanova, M.; Atanassov, I.; Kozáková, Z.; Krčma, F. Microwave Plasma Torch Generated in Argon for Small Berries Surface Treatment. Appl. Sci. 2018, 8, 1870. [Google Scholar] [CrossRef] [Green Version]
- Narimisa, M.; Krčma, F.; Onyshchenko, Y.; Kozáková, Z.; Morent, R.; De Geyter, N. Atmospheric Pressure Microwave Plasma Jet for Organic Thin Film Deposition. Polymers 2020, 12, 354. [Google Scholar] [CrossRef] [Green Version]
- Lebedev, Y.A. Microwave Discharges at Low Pressures and Peculiarities of the Processes in Strongly Non-Uniform Plasma. Plasma Sources Sci. Technol. 2015, 24, 53001. [Google Scholar] [CrossRef]
- Fidel, P.L.; Vazquez, J.A.; Sobel, J.D. Candida glabrata: Review of Epidemiology, Pathogenesis, and Clinical Disease with Comparison to C. albicans. Clin. Microbiol. Rev. 1999, 12, 80–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, K.M.; Kokošar, J.; Guo, X.; Gu, Z.; Ishchuk, O.P.; Piškur, J. Genome Structure and Dynamics of the Yeast Pathogen Candida Glabrata. Fems Yeast Res. 2014, 14, 529–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes-Giannini, M.J.S.; Bernardi, T.; Scorzoni, L.; Fusco-Almeida, A.M.; Sardi, J.C.O. Candida Species: Current Epidemiology, Pathogenicity, Biofilm Formation, Natural Antifungal Products and New Therapeutic Options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar]
- Sykes, J.E. Candidiasis. Canine Feline Infect. Dis. 2014, 67, 653–659. [Google Scholar]
- Rodrigues, C.; Rodriguez, M.; Silva, S.; Henriques, M.; Ishchuk, O.P.; Piškur, J. Candida glabrata Biofilms: How Far Have we Come? J. Fungi 2017, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Czech Microorganism Collection. Available online: https://www.sci.muni.cz/ccm/index.html (accessed on 6 August 2020).











| Time of Application [s] | Undiluted Culture | 10-Fold Diluted Culture | 100-Fold Diluted Culture | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Power | 9 W | 12 W | 9 W | 12 W | 9 W | 12 W | ||||
| Incubation | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 48 h | 48 h |
| 15 | A | A | B | B | A | A | A, B | A, B | A | B |
| 30 | B | B | C | C | B | B | C, D | C | B | B, C |
| 60 | C | B, C | D, E | D | C, D | C | D | C, D | C, D | C |
| 120 | D | D | E | C, D | D | C, D | D, E | C, D | C | D |
| 300 | G | F | - | - | - | - | F | E | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trebulová, K.; Krčma, F.; Kozáková, Z.; Matoušková, P. Impact of Microwave Plasma Torch on the Yeast Candida glabrata. Appl. Sci. 2020, 10, 5538. https://doi.org/10.3390/app10165538
Trebulová K, Krčma F, Kozáková Z, Matoušková P. Impact of Microwave Plasma Torch on the Yeast Candida glabrata. Applied Sciences. 2020; 10(16):5538. https://doi.org/10.3390/app10165538
Chicago/Turabian StyleTrebulová, Kristína, František Krčma, Zdenka Kozáková, and Petra Matoušková. 2020. "Impact of Microwave Plasma Torch on the Yeast Candida glabrata" Applied Sciences 10, no. 16: 5538. https://doi.org/10.3390/app10165538
APA StyleTrebulová, K., Krčma, F., Kozáková, Z., & Matoušková, P. (2020). Impact of Microwave Plasma Torch on the Yeast Candida glabrata. Applied Sciences, 10(16), 5538. https://doi.org/10.3390/app10165538