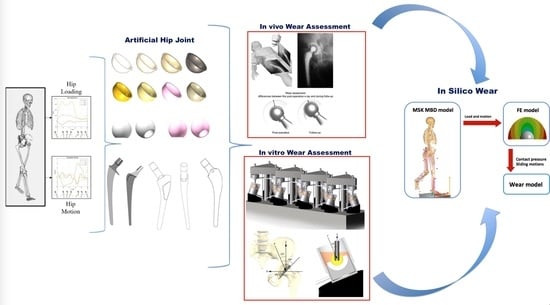
A Perspective on Biotribology in Arthroplasty: From In Vitro toward the Accurate In Silico Wear Prediction
Abstract
:1. Introduction
2. In Vivo Wear Testing
3. In Vitro Wear Testing
- The so-called quick tests, which provide information exclusively on the intrinsic features of the materials studied. These wear tests can be performed in dry and lubricated conditions and in different configurations. Measuring friction, wear, and material mechanical properties is a way for engineers to understand how materials will stand up to the rigors of biomedical application. These wear tests are fast and cheap but, unfortunately, they do not accurately reproduce the contact geometries and the kinematics of the real prostheses [29]. A schematic representation is given in Figure 2.
- Vice versa, the hip and knee joint wear simulators are machines in which real prostheses are mounted on these apparatuses, as intended in vivo, and tested in an environment that simulates physiological conditions. These simulators can reproduce a simplified level of walking, as specified in international guidelines [30,31]. Recently, efforts are devoted to the simulation of a more demanding task that would reproduce the wear rates of daily living activities [32,33]. A schematic representation is given in Figure 3.
4. Toward the In Silico Wear Test
5. Research Directions
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rizzoli, I.O. REPORT of R.I.P.O. Regional Register of Orthopaedic Prosthetic Implantology Overall Data Hip, Knee and Shoulder Arthroplasty in Emilia-Romagna Region (Italy) 2000–2017. Available online: https://ripo.cineca.it/authzssl/pdf/report_eng_2017.pdf (accessed on 7 September 2020).
- Viceconti, M.; Affatato, S.; Baleani, M.; Bordini, B.; Cristofolini, L.; Taddei, F. Pre-clinical validation of joint prostheses: A systematic approach. J. Mech. Behav. Biomed. Mater. 2009, 2, 120–127. [Google Scholar] [CrossRef]
- Barrère, F.; Mahmood, T.A.; de Groot, K.; van Blitterswijk, C.A. Advanced biomaterials for skeletal tissue regeneration: Instructive and smart functions. Mater. Sci. Eng. R Rep. 2008, 59, 38–71. [Google Scholar] [CrossRef]
- ISO-10993 Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process; ISO International: Geneva, Switzerland, 2018.
- Boyer, B.; Bordini, B.; Caputo, D.; Neri, T.; Stea, S.; Toni, A. What are the influencing factors on hip and knee arthroplasty survival? Prospective cohort study on 63619 arthroplasties. Orthop. Traumatol. Surg. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Affatato, S.; Cosentino, M.; Castagnini, F.; Bordini, B. Registry study on failure incidence in 1,127 revised hip implants with stem trunnion re-use after 10 years of follow-up: Limited influence of an adapter sleeve. Acta Orthop. 2019, 90, 417–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javierre, E.; Moreo, P.; Doblaré, M.; Garcia Aznar, J.M. Numerical modeling of a mechano-chemical theory for wound contraction analysis. Int. J. Solids Struct. 2009, 46, 3597–3606. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, K.A.; Chatzikalymniou, A.P.; Skinner, F.K. Combining Theory, Model, and Experiment to Explain How Intrinsic Theta Rhythms Are Generated in an In Vitro Whole Hippocampus Preparation without Oscillatory Inputs. eNeuro 2017, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grillini, L.L.; Affatato, S. How to measure wear following total hip arthroplasty. Hip Int. 2013, 23, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Banaszkiewicz, P.A. Effect of femoral head size on wear of the polyethylene acetabular component. In Classic Papers in Orthopaedics; Springer: Berlin, Germany, 2014; ISBN 9781447154518. [Google Scholar]
- Livermore, J.; Ilstrup, D.; Morrey, B. Effect of femoral head size on wear of the polyethylene acetabular component. JBJS 1990, 72, 518–528. [Google Scholar] [CrossRef]
- Kabo, J.M.; Gebhard, J.S.; Loren, G.; Amstutz, H.C. In vivo wear of polyethylene acetabular components. J. Bone Jt. Surg. 1993, 75, 254–258. [Google Scholar] [CrossRef] [Green Version]
- Sofka, C.M.; Potter, H.G.; Figgie, M.; Laskin, R. Magnetic Resonance Imaging of Total Knee Arthroplasty. Clin. Orthop. Relat. Res. 2003, 129–135. [Google Scholar] [CrossRef]
- West, J.; Romu, T.; Thorell, S.; Lindblom, H.; Berin, E.; Holm, A.C.S.; Åstrand, L.L.; Karlsson, A.; Borga, M.; Hammar, M.; et al. Precision of MRI-based body composition measurements of postmenopausal women. PLoS ONE 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellmanns, K.; McBean, K.; Thoirs, K. Magnetic Resonance Imaging in the measurement of whole body muscle mass: A comparison of interval gap methods. Radiography 2015, 21, e35–e39. [Google Scholar] [CrossRef]
- Brinke, B.T.E.N.; Beumer, A.; Koenraadt, K.L.M.; Eygendaal, D.; Kraan, G.A.; Mathijssen, N.M.C. The accuracy and precision of radiostereometric analysis in upper limb arthroplasty—A systematic review of 23 RSA studies. Acta Orthop. 2017, 88, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Holm-Glad, T.; Reigstad, O.; Tsukanaka, M.; Røkkum, M.; Ro€hrl, S.M. High Precision and Accuracy of Model-Based RSA for Analysis of Wrist Arthroplasty. J. Orthop. Res. 2018, 36, 3053–3063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Önsten, I.; Berzins, A.; Shott, S.; Sumner, D.R. Accuracy and precision of radiostereometric analysis in the measurement of THR femoral component translations: Human and canine in vitro models. J. Orthop. Res. 2001, 19, 1162–1167. [Google Scholar] [CrossRef]
- Ranstam, J.; Ryd, L.; Onsten, I. Accurate accuracy assessment: Review of basic principles. Acta Orthop. Scand. 2000, 71, 106–108. [Google Scholar] [CrossRef]
- Seehaus, F.; Emmerich, J.; Kaptein, B.L.; Windhagen, H.; Hurschler, C. Dependence of model-based RSA accuracy on higher and lower implant surface model quality. Biomed. Eng. Online 2013, 12, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Selvik, G. A stereophotogrammetric system for the study of human movements. Scand. J. Rehabil Med. Suppl. 1978, 6, 16–20. [Google Scholar]
- Johnston, C.; Kerr, J.; Ford, S.; O’Byrne, J.; Eustace, S. MRI as a problem-solving tool in unexplained failed total hip replacement following conventional assessment. Skelet. Radiol. 2007. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.L.; Schmier, J.; Mowat, F.; Saleh, K.; Dybvik, E.; Kärrholm, J.; Garellick, G.; Havelin, L.I.; Furnes, O.; et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J. Bone Jt. Surg. Am. 2007, 89 (Suppl. 3), 144–151. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic burden of periprosthetic joint infection in the united states. J. Arthroplast. 2012. [Google Scholar] [CrossRef] [PubMed]
- Dowson, D. History of Tribology, 2nd ed.; Wiley Blackwell: London, UK, 1998. [Google Scholar]
- Collins English Dictionary—Complete and Unabridged Invitro.pdf. Available online: https://www.thefreedictionary.com/in+vitro (accessed on 6 May 2020).
- Affatato, S.; Spinelli, M.; Zavalloni, M.; Mazzega-Fabbro, C.; Viceconti, M. Tribology and total hip joint replacement: Current concepts in mechanical simulation. Med. Eng. Phys. 2008, 30, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- ASTM F2003. ASTM F2003-02(2015) Standard Practice for Accelerated Aging of Ultra-High. Molecular Weight Polyethylene after Gamma Irradiation in Air; ASTM International: Conshohocken, PA, USA, 2003. [Google Scholar]
- ISO DIS 14242-1 Implants for Surgery—Wear of Total Hip-Joint Prostheses—Part 1: Loading and Displacement Paramenters for Wear-Testing Machines and Corresponding Environmental Conditions for Test; ISO International: Geneva, Switzerland, 2012.
- ISO-4243/3. ISO ISO 14243-3:2014(en), Implants for Surgery—Wear of Total Knee-Joint Prostheses—Part 3: Loading and Displacement Parameters for Wear-Testing Machines with Displacement Control and Corresponding Environmental Conditions for Test; ISO International: Geneva, Switzerland, 2004. [Google Scholar]
- Abdel-Jaber, S.; Belvedere, C.; De Mattia, J.S.; Leardini, A.; Affatato, S. A new protocol for wear testing of total knee prostheses from real joint kinematic data: Towards a scenario of realistic simulations of daily living activities. J. Biomech. 2016, 49. [Google Scholar] [CrossRef] [PubMed]
- Affatato, S. Towards wear testing of high demanding.pdf. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 260–266. [Google Scholar] [CrossRef]
- Brockett, C.; Williams, S.; Jin, Z.; Isaac, G.; Fisher, J. Friction of Total Hip Replacements With Different Bearings and Loading Conditions. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 81, 508–515. [Google Scholar] [CrossRef]
- Dowson, D. New joints for the Millennium: Wear control in total replacement hip joints. Proc. Inst. Mech. Eng. H 2001, 215, 335–358. [Google Scholar] [CrossRef]
- Affatato, S.; Zanini, F.; Carmignato, S. Micro X-ray computed tomography mass loss assessment of different UHMWPE: A hip joint simulator study on standard vs. cross-linked polyethylene. PLoS ONE 2017, 12, e0170263. [Google Scholar] [CrossRef] [Green Version]
- Affatato, S.; Valigi, M.C.; Logozzo, S. Wear distribution detection of knee joint prostheses by means of 3D optical scanners. Materials 2017, 10, 364. [Google Scholar] [CrossRef]
- Tunev, S.S. Differences between in Vitro, in Vivo, and in Silico Studies in Vitro Studies in Vivo Studies in Silico Studies Read. The Marshall Protocol. Autoimmunity Research Foundation. Available online: https://mpkb.org/home/patients/assessing_literature/in_vitro_studies (accessed on 7 September 2020).
- Sirakoulis, G.C.; Karafyllidis, I.; Mizas, C.; Mardiris, V.; Thanailakis, A.; Tsalides, P. A cellular automaton model for the study of DNA sequence evolution. Comput. Biol. Med. 2003, 33, 439–453. [Google Scholar] [CrossRef]
- Sieburg, H.B. Physiological studies in silico. In Lectures in Complex Systems, SFI Studies in the Sciences of Complexity; Nadel, L., Stein, D., Eds.; Addison-Wesley: San Diego, CA, USA, 1991; Volume 3, pp. 367–390. [Google Scholar]
- Ruggiero, A.; Sicilia, A. Lubrication modeling and wear calculation in artificial hip joint during the gait. Tribol. Int. 2020, 142, 1–16. [Google Scholar] [CrossRef]
- Ruggiero, A.; D’Amato, R.; Affatato, S. Comparison of meshing strategies in THR finite element modelling. Materials 2019, 12, 2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggiero, A.; Merola, M.; Affatato, S. Finite element simulations of hard-on-soft hip joint prosthesis accounting for dynamic loads calculated from a Musculoskeletal model during walking. Materials 2018, 11, 574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Affatato, S.; Merola, M.; Ruggiero, A. Development of a novel in silico model to investigate the influence of radial clearance on the acetabular cup contact pressure in hip implants. Materials 2018, 11, 1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Jaber, S.; Belvedere, C.; Leardini, A.; Affatato, S. Wear simulation of total knee prostheses using load and kinematics waveforms from stair climbing. J. Biomech. 2015, 48, 3830–3836. [Google Scholar] [CrossRef]
- Ruggiero, A.; Sicilia, A.; Affatato, S. In silico total hip replacement wear testing in the framework of ISO 14242-3 accounting for mixed elasto-hydrodynamic lubrication effects. Wear 2020, 460–461, 203420. [Google Scholar] [CrossRef]
- Azpiroz-Zabala, M.; Storms, J.; Van Der Vegt, H.; Walstra, D. A numerical model of a 3-dimensional low-density turbidity current in the deep ocean: Testing hypotheses on turbidity currents in deep detail. Geophys. Res. Abstr. 2019, 21, 8459. [Google Scholar]
- Christie, M.J.; Barrington, S.A.; Brinson, M.F.; Ruhling, M.E.; DeBoer, D.K. Bridging massive acetabular defects with the triflange cup: 2- to 9-year results. Clin. Orthop. Relat. Res. 2001, 393, 216–227. [Google Scholar] [CrossRef]
- Ruggiero, A. Milestones in natural lubrication of synovial joints. Front. Mech. Eng. 2020, 10. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and rubbing of flate surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Mazzucco, D.; Spector, M. Effects of contact area and stress on the volumetric wear of ultrahigh molecular weight polyethylene. Wear 2003, 254, 514–522. [Google Scholar] [CrossRef]
- Kang, L.; Galvin, A.L.; Fisher, J.; Jin, J. Enhanced computational prediction of polyethylene wear in hip joints by incorporating cross-shear and contact pressure in additional to load and sliding distance: Effect of head diameter. J. Biomech. 2009, 42, 912–918. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.T.; Luo, Y.; Brandt, J.M. In-vitro and in-silico investigations on the influence of contact pressure on cross-linked polyethylene wear in total knee replacements. Wear 2015. [Google Scholar] [CrossRef]
- O’Brien, S.T.; Bohm, E.R.; Petrak, M.J.; Wyss, U.P.; Brandt, J.-M. An energy dissipation and cross shear time dependent computational wear model for the analysis of polyethylene wear in total knee replacements. J. Biomech. 2014. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Asay, D.B.; Dugger, M.T. Nanotribology and MEMS. Nano Today 2007, 2, 22–29. [Google Scholar] [CrossRef]
- Bhushan, B. Nanotribology and nanomechanics. Wear 2005. [Google Scholar] [CrossRef]
- Tzanakis, I.; Hadfield, M.; Thomas, B.; Noya, S.M.; Henshaw, I.; Austen, S. Future perspectives on sustainable tribology. Renew. Sustain. Energy Rev. 2012, 16, 4126–4140. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.R.; Jin, Z.M. Biotribology: Recent Progresses and Future Perspectives. Biosurface Biotribology 2015, 1, 3–24. [Google Scholar] [CrossRef] [Green Version]
- Cann, P.; Wimmer, M. Welcome to the first issue of Biotribology. Biotribology 2015. [Google Scholar] [CrossRef]
- Martin, J.M.; Donnet, C.; Le Mogne, T.; Epicier, T. Superlubricity of molybdenum disulphide. Phys. Rev. B 1993. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, X.; Ma, T.; Liu, D.; Gao, L.; Li, X.; Zhang, J.; Hu, Y.; Wang, H.; Dai, Y.; et al. Superlubricity of a graphene/MoS2 heterostructure: A combined experimental and DFT study. Nanoscale 2017. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.; Deshmukh, S.A.; Sankaranarayanan, S.K.R.S.; Erdemir, A.; Sumant, A.V. Macroscale superlubricity enabled by graphene nanoscroll formation. Science 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vakis, A.; Yastrebov, V.; Scheibert, J.; Minfray, C.; Nicola, L.; Dini, D.; Almqvist, A.; Paggi, M.; Lee, S.; Limbert, G.; et al. Modeling and simulation in tribology across scales: An overview. Tribol. Int. 2018, 125, 1–83. [Google Scholar] [CrossRef]
- Morrison, T.M.; Pathmanathan, P.; Adwan, M.; Margerrison, E. Advancing regulatory science with computational modeling for medical devices at the FDA’s office of science and engineering laboratories. Front. Med. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- TMorrison, T.M. Innovations in modeling and simulation: Patient-centered healthcare. Ann. Biomed. Eng. 2016, 44, 3719–3721. Available online: http://rug.on.worldcat.org/atoztitles/link/?sid=EMBASE&issn=15739686&id=doi:10.1007%2Fs10439-016-1710-7&atitle=Innovations+in+modeling+and+simulation%3A+Patient-centered+healthcare&stitle=Ann+Biomed+Eng&title=Annals+of+Biomedical+Engineering&volume=44&issue=12&spage=3719&epage=3721&aulast=Morrison&aufirst=Tina+M.&auinit=T.M.&aufull=Morrison+T.M.&coden=&isbn=&pages=3719-3721&date=2016&auinit1=T&auinitm=M (accessed on 7 September 2020). [CrossRef]
- Ruggiero, A.; Zhang, H. Editorial: Biotribology and Biotribocorrosion Properties of Implantable Biomaterials. Front. Mech. Eng. 2020, 6. [Google Scholar] [CrossRef]
- Shi, L.; Guo, Z.; Liu, W. The recent progress of tribological biomaterials. Biosurface Biotribol. 2015, 26. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Affatato, S.; Ruggiero, A. A Perspective on Biotribology in Arthroplasty: From In Vitro toward the Accurate In Silico Wear Prediction. Appl. Sci. 2020, 10, 6312. https://doi.org/10.3390/app10186312
Affatato S, Ruggiero A. A Perspective on Biotribology in Arthroplasty: From In Vitro toward the Accurate In Silico Wear Prediction. Applied Sciences. 2020; 10(18):6312. https://doi.org/10.3390/app10186312
Chicago/Turabian StyleAffatato, Saverio, and Alessandro Ruggiero. 2020. "A Perspective on Biotribology in Arthroplasty: From In Vitro toward the Accurate In Silico Wear Prediction" Applied Sciences 10, no. 18: 6312. https://doi.org/10.3390/app10186312
APA StyleAffatato, S., & Ruggiero, A. (2020). A Perspective on Biotribology in Arthroplasty: From In Vitro toward the Accurate In Silico Wear Prediction. Applied Sciences, 10(18), 6312. https://doi.org/10.3390/app10186312