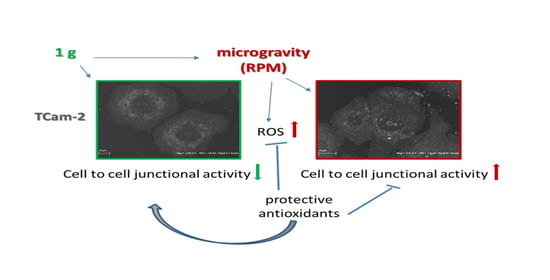
Microgravity-Induced Cell-to-Cell Junctional Contacts Are Counteracted by Antioxidant Compounds in TCam-2 Seminoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Equipment and Cell Exposure Parameters
2.2. Cell Cultures
2.3. Electron Microscopy
2.3.1. Scanning Electron Microscopy (SEM) Analysis
2.3.2. Transmission Electron Microscopy (TEM) Analysis
2.4. Confocal Microscopy Analyses
2.4.1. Immunofluorescence
2.4.2. F-Actin Staining
2.5. Western Blotting
2.6. Statistical Analyses
2.7. Data Availability Statement
3. Results
3.1. Simulated Microgravity Influences the TCam-2 Cell Membrane Surface at Cell-to-Cell Contacts
3.2. Simulated Microgravity Influences the TCam-2 Cell Ultrastructure at the Junctional Level
3.3. Simulated-Microgravity Exposure Modifies the TCam-2 Distribution Pattern of Cadherins but Does Not Influence Beta1 Integrin and Vimentin Localization
3.4. Antioxidant Compounds Counteract the RPM-Induced Junctional Contacts
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Locatelli, L.; Cazzaniga, A.; De Palma, C.; Castiglioni, S.; Maier, J.A.M. Mitophagy contributes to endothelial adaptation to simulated microgravity. FASEB J. 2020, 34, 1833–1845. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhong, G.; Zhou, Y.; Yang, Y.; Tan, Y.; Li, Y.; Gao, X.; Sun, W.; Li, J.; Jin, X.; et al. Alteration of calcium signalling in cardiomyocyte induced by simulated microgravity and hypergravity. Cell Prolif. 2020, 53, e12783. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, H.; Zhang, F.; Dong, X.; Hao, T.; Jiang, X.; Zheng, W.; Zhang, T.; Chen, X.; Wang, P.; et al. Spaceflight Promoted Myocardial Differentiation of Induced Pluripotent Stem Cells: Results from Tianzhou-1 Space Mission. Stem Cells Dev. 2019, 28, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Wnorowski, A.; Sharma, A.; Chen, H.; Wu, H.; Shao, N.Y.; Sayed, N.; Liu, C.; Countryman, S.; Stodieck, L.S.; Rubins, K.H.; et al. Effects of Spaceflight on Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Structure and Function. Stem Cell Rep. 2019, 13, 960–969. [Google Scholar] [CrossRef]
- Bradbury, P.; Wu, H.; Choi, J.U.; Rowan, A.E.; Zhang, H.; Poole, K.; Lauko, J.; Chou, J. Modeling the Impact of Microgravity at the Cellular Level: Implications for Human Disease. Front. Cell Dev. Biol. 2020, 8, 96. [Google Scholar] [CrossRef]
- Morabito, C.; Guarnieri, S.; Cucina, A.; Bizzarri, M.; Mariggio, M.A. Antioxidant Strategy to Prevent Simulated Microgravity-Induced Effects on Bone Osteoblasts. Int. J. Mol. Sci. 2020, 21, 3638. [Google Scholar] [CrossRef]
- Nassef, M.Z.; Kopp, S.; Wehland, M.; Melnik, D.; Sahana, J.; Kruger, M.; Corydon, T.J.; Oltmann, H.; Schmitz, B.; Schutte, A.; et al. Real Microgravity Influences the Cytoskeleton and Focal Adhesions in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3156. [Google Scholar] [CrossRef]
- Nassef, M.Z.; Kopp, S.; Melnik, D.; Corydon, T.J.; Sahana, J.; Kruger, M.; Wehland, M.; Bauer, T.J.; Liemersdorf, C.; Hemmersbach, R.; et al. Short-Term Microgravity Influences Cell Adhesion in Human Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 5730. [Google Scholar] [CrossRef]
- Bizzarri, M.; Monici, M.; van Loon, J.J. How microgravity affects the biology of living systems. BioMed Res. Int. 2015, 2015, 863075. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, Z.; Liang, M.; Luo, F.; Xu, J.; Dou, C.; Dong, S. The role of physical forces in osteoclastogenesis. J. Cell Physiol. 2019, 234, 12498–12507. [Google Scholar] [CrossRef]
- Po, A.; Giuliani, A.; Masiello, M.G.; Cucina, A.; Catizone, A.; Ricci, G.; Chiacchiarini, M.; Tafani, M.; Ferretti, E.; Bizzarri, M. Phenotypic transitions enacted by simulated microgravity do not alter coherence in gene transcription profile. NPJ Microgravity 2019, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Morabito, C.; Lanuti, P.; Caprara, G.A.; Marchisio, M.; Bizzarri, M.; Guarnieri, S.; Mariggio, M.A. Physiological Responses of Jurkat Lymphocytes to Simulated Microgravity Conditions. Int. J. Mol. Sci. 2019, 20, 1892. [Google Scholar] [CrossRef]
- Nassef, M.Z.; Melnik, D.; Kopp, S.; Sahana, J.; Infanger, M.; Lutzenberg, R.; Relja, B.; Wehland, M.; Grimm, D.; Kruger, M. Breast Cancer Cells in Microgravity: New Aspects for Cancer Research. Int. J. Mol. Sci. 2020, 21, 7345. [Google Scholar] [CrossRef]
- Masiello, M.G.; Verna, R.; Cucina, A.; Bizzarri, M. Physical constraints in cell fate specification. A case in point: Microgravity and phenotypes differentiation. Prog. Biophys. Mol. Biol. 2018, 134, 55–67. [Google Scholar] [CrossRef]
- Di Agostino, S.; Botti, F.; Di Carlo, A.; Sette, C.; Geremia, R. Meiotic progression of isolated mouse spermatocytes under simulated microgravity. Reproduction 2004, 128, 25–32. [Google Scholar] [CrossRef]
- Ricci, G.; Catizone, A.; Esposito, R.; Galdieri, M. Microgravity effect on testicular functions. J. Gravit. Physiol. 2004, 11, P61–P62. [Google Scholar]
- Ricci, G.; Esposito, R.; Catizone, A.; Galdieri, M. Direct effects of microgravity on testicular function: Analysis of hystological, molecular and physiologic parameters. J. Endocrinol. Investig. 2008, 31, 229–237. [Google Scholar] [CrossRef]
- Pellegrini, M.; Di Siena, S.; Claps, G.; Di Cesare, S.; Dolci, S.; Rossi, P.; Geremia, R.; Grimaldi, P. Microgravity promotes differentiation and meiotic entry of postnatal mouse male germ cells. PLoS ONE 2010, 5, e9064. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhang, H.; Miao, G.Y.; Xie, Y.; Sun, C.; Di, C.X.; Liu, Y.; Liu, Y.Y.; Zhang, X.; Ma, X.F.; et al. Simulated microgravity conditions and carbon ion irradiation induce spermatogenic cell apoptosis and sperm DNA damage. Biomed. Environ. Sci. 2013, 26, 726–734. [Google Scholar] [CrossRef]
- Strollo, F.; Masini, M.A.; Pastorino, M.; Ricci, F.; Vadrucci, S.; Cogoli-Greuter, M.; Uva, B.M. Microgravity-induced alterations in cultured testicular cells. J. Gravit. Physiol. 2004, 11, P187–P188. [Google Scholar]
- Nowacki, D.; Klinger, F.G.; Mazur, G.; De Felici, M. Effect of Culture in Simulated Microgravity on the Development of Mouse Embryonic Testes. Adv. Clin. Exp. Med. 2015, 24, 769–774. [Google Scholar] [CrossRef]
- de Jong, J.; Stoop, H.; Gillis, A.J.; Hersmus, R.; van Gurp, R.J.; van de Geijn, G.J.; van Drunen, E.; Beverloo, H.B.; Schneider, D.T.; Sherlock, J.K.; et al. Further characterization of the first seminoma cell line TCam-2. Genes Chromosomes Cancer 2008, 47, 185–196. [Google Scholar] [CrossRef]
- Eckert, D.; Nettersheim, D.; Heukamp, L.C.; Kitazawa, S.; Biermann, K.; Schorle, H. TCam-2 but not JKT-1 cells resemble seminoma in cell culture. Cell Tissue Res. 2008, 331, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Gotoh, A.; Kamidono, S.; Kitazawa, S. [Establishment and characterization of a new human testicular germ cell tumor cell line (TCam-2)]. Nihon Hinyokika Gakkai Zasshi 1993, 84, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Ferranti, F.; Caruso, M.; Cammarota, M.; Masiello, M.G.; Corano Scheri, K.; Fabrizi, C.; Fumagalli, L.; Schiraldi, C.; Cucina, A.; Catizone, A.; et al. Cytoskeleton modifications and autophagy induction in TCam-2 seminoma cells exposed to simulated microgravity. BioMed Res. Int. 2014, 2014, 904396. [Google Scholar] [CrossRef] [PubMed]
- Morabito, C.; Guarnieri, S.; Catizone, A.; Schiraldi, C.; Ricci, G.; Mariggio, M.A. Transient increases in intracellular calcium and reactive oxygen species levels in TCam-2 cells exposed to microgravity. Sci. Rep. 2017, 7, 15648. [Google Scholar] [CrossRef] [PubMed]
- van Roy, F. Beyond E-cadherin: Roles of other cadherin superfamily members in cancer. Nat. Rev. Cancer 2014, 14, 121–134. [Google Scholar] [CrossRef]
- Casal, J.I.; Bartolome, R.A. Beyond N-Cadherin, Relevance of Cadherins 5, 6 and 17 in Cancer Progression and Metastasis. Int. J. Mol. Sci. 2019, 20, 3373. [Google Scholar] [CrossRef]
- Ferranti, F.; D’Anselmi, F.; Caruso, M.; Lei, V.; Dinicola, S.; Pasqualato, A.; Cucina, A.; Palombo, A.; Ricci, G.; Catizone, A.; et al. TCam-2 seminoma cells exposed to egg-derived microenvironment modify their shape, adhesive pattern and migratory behaviour: A molecular and morphometric analysis. PLoS ONE 2013, 8, e76192. [Google Scholar] [CrossRef]
- Lim, S.O.; Gu, J.M.; Kim, M.S.; Kim, H.S.; Park, Y.N.; Park, C.K.; Cho, J.W.; Park, Y.M.; Jung, G. Epigenetic changes induced by reactive oxygen species in hepatocellular carcinoma: Methylation of the E-cadherin promoter. Gastroenterology 2008, 135, 2128–2140, 2140.e1–2140.e8. [Google Scholar] [CrossRef]
- Yeung, K.T.; Yang, J. Epithelial-mesenchymal transition in tumor metastasis. Mol. Oncol. 2017, 11, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Jeong, A.J.; Kim, Y.J.; Lim, M.H.; Lee, H.; Noh, K.; Kim, B.H.; Chung, J.W.; Cho, C.H.; Kim, S.; Ye, S.K. Microgravity induces autophagy via mitochondrial dysfunction in human Hodgkin’s lymphoma cells. Sci. Rep. 2018, 8, 14646. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Uchida, T.; Yoshie, T.; Mizote, Y.; Ishikawa, F.; Katsuyama, M.; Shibanuma, M. A mitochondrial ROS pathway controls matrix metalloproteinase 9 levels and invasive properties in RAS-activated cancer cells. FEBS J. 2019, 286, 459–478. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.; An, L.; Fan, Y.; Hang, H.; Wang, S. Simulated microgravity potentiates generation of reactive oxygen species in cells. Biophys. Rep. 2016, 2, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, Z.; Niu, B.; Zhang, J.; Tan, T.K.; Lee, S.R.; Zhao, Y.; Harris, D.C.; Zheng, G. E-cadherin/beta-catenin complex and the epithelial barrier. J. Biomed. Biotechnol. 2011, 2011, 567305. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, K.; Wei, D.; Tian, Y.; Gao, Y.; Chen, Z.; Qian, A. The Impact of Spaceflight and Simulated Microgravity on Cell Adhesion. Int. J. Mol. Sci. 2020, 21, 3031. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Bai, Y.; Shi, R.; Wei, H.; Zhang, S. Mouse undifferentiated spermatogonial stem cells cultured as aggregates under simulated microgravity. Andrologia 2014, 46, 1013–1021. [Google Scholar] [CrossRef]
- Ranieri, D.; Proietti, S.; Dinicola, S.; Masiello, M.G.; Rosato, B.; Ricci, G.; Cucina, A.; Catizone, A.; Bizzarri, M.; Torrisi, M.R. Simulated microgravity triggers epithelial mesenchymal transition in human keratinocytes. Sci. Rep. 2017, 7, 538. [Google Scholar] [CrossRef]
- Motabagani, M.A. Morphological and morphometric study on the effect of simulated microgravity on rat testis. Chin. J. Physiol. 2007, 50, 199–209. [Google Scholar]
- Zetter, B.R. Adhesion molecules in tumor metastasis. Semin Cancer Biol. 1993, 4, 219–229. [Google Scholar]
- Cavallaro, U.; Christofori, G. Multitasking in tumor progression: Signaling functions of cell adhesion molecules. Ann. N. Y. Acad Sci. 2004, 1014, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Honecker, F.; Kersemaekers, A.M.; Molier, M.; Van Weeren, P.C.; Stoop, H.; De Krijger, R.R.; Wolffenbuttel, K.P.; Oosterhuis, W.; Bokemeyer, C.; Looijenga, L.H. Involvement of E-cadherin and beta-catenin in germ cell tumours and in normal male fetal germ cell development. J. Pathol. 2004, 204, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Buken, C.; Sahana, J.; Corydon, T.J.; Melnik, D.; Bauer, J.; Wehland, M.; Kruger, M.; Balk, S.; Abuagela, N.; Infanger, M.; et al. Morphological and Molecular Changes in Juvenile Normal Human Fibroblasts Exposed to Simulated Microgravity. Sci. Rep. 2019, 9, 11882. [Google Scholar] [CrossRef]
- Ratushnyy, A.; Ezdakova, M.; Yakubets, D.; Buravkova, L. Angiogenic Activity of Human Adipose-Derived Mesenchymal Stem Cells Under Simulated Microgravity. Stem Cells Dev. 2018, 27, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Milisav, I. The Role of Antioxidants in Cancer, Friends or Foes? Curr. Pharm. Des. 2018, 24, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Lutsenko, S.V.; Terentiev, A.A. Reactive Oxygen and Nitrogen Species-Induced Protein Modifications: Implication in Carcinogenesis and Anticancer Therapy. Cancer Res. 2018, 78, 6040–6047. [Google Scholar] [CrossRef] [PubMed]
- Sedlackova, L.; Korolchuk, V.I. The crosstalk of NAD, ROS and autophagy in cellular health and ageing. Biogerontology 2020, 21, 381–397. [Google Scholar] [CrossRef]
- Yun, J.; Finkel, T. Mitohormesis. Cell Metab. 2014, 19, 757–766. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015, 33, 8–13. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. TOR signaling couples oxygen sensing to lifespan in C. elegans. Cell Rep. 2014, 9, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S. Mitochondria as signaling organelles. BMC Biol. 2014, 12, 34. [Google Scholar] [CrossRef]
- Chiarugi, P. Reactive oxygen species as mediators of cell adhesion. Ital. J. Biochem. 2003, 52, 28–32. [Google Scholar] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catizone, A.; Morabito, C.; Cammarota, M.; Schiraldi, C.; Corano Scheri, K.; Ferranti, F.; Mariggiò, M.A.; Ricci, G. Microgravity-Induced Cell-to-Cell Junctional Contacts Are Counteracted by Antioxidant Compounds in TCam-2 Seminoma Cells. Appl. Sci. 2020, 10, 8289. https://doi.org/10.3390/app10228289
Catizone A, Morabito C, Cammarota M, Schiraldi C, Corano Scheri K, Ferranti F, Mariggiò MA, Ricci G. Microgravity-Induced Cell-to-Cell Junctional Contacts Are Counteracted by Antioxidant Compounds in TCam-2 Seminoma Cells. Applied Sciences. 2020; 10(22):8289. https://doi.org/10.3390/app10228289
Chicago/Turabian StyleCatizone, Angela, Caterina Morabito, Marcella Cammarota, Chiara Schiraldi, Katia Corano Scheri, Francesca Ferranti, Maria A. Mariggiò, and Giulia Ricci. 2020. "Microgravity-Induced Cell-to-Cell Junctional Contacts Are Counteracted by Antioxidant Compounds in TCam-2 Seminoma Cells" Applied Sciences 10, no. 22: 8289. https://doi.org/10.3390/app10228289
APA StyleCatizone, A., Morabito, C., Cammarota, M., Schiraldi, C., Corano Scheri, K., Ferranti, F., Mariggiò, M. A., & Ricci, G. (2020). Microgravity-Induced Cell-to-Cell Junctional Contacts Are Counteracted by Antioxidant Compounds in TCam-2 Seminoma Cells. Applied Sciences, 10(22), 8289. https://doi.org/10.3390/app10228289