Changes in Growth and Production of Non-Psychotropic Cannabinoids Induced by Pre-Sowing Treatment of Hemp Seeds with Cold Plasma, Vacuum and Electromagnetic Field
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
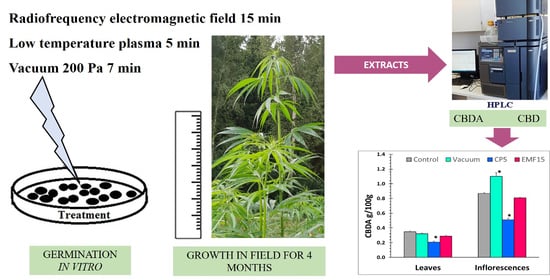
2.2. Seed Treatment with CP, Vacuum, and EMF
2.3. Seed Germination Tests
2.4. Plant Cultivation in the Field and Morphometric Analysis
2.5. Measurement of Radical Scavenging Activity
2.6. Detection of Cannabinoid Amount
2.7. Statistical Analysis
3. Results
3.1. Effects on In Vitro Germination
3.2. Changes in Growth of Female and Male Plants
3.3. Changes in the Content of CBDA (CBD) and in Radical Scavenging Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ewel, J.J.; Schreeg, L.A.; Sinclair, T.R. Resources for crop production: Accessing the unavailable. Trends Plant Sci. 2018, 24, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Broun, P.; Cakmak, I.; Condon, L.; Fedoroff, N.; Gonzalez-Valero, J.; Graham, I.; Lewis, J.; Moloney, M.; Oniang’o, R.K.; et al. Planting seeds for the future of food. J. Sci. Food Agric. 2016, 96, 1409–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and, Evolution of Dormancy and Germination, 2nd ed.; Academic Press: Lexington, KY, USA, 2014; pp. 101–116. [Google Scholar]
- Chahtane, H.; Kim, W.; Lopez-Molina, L. Primary seed dormancy: A temporally multilayered riddle waiting to be unlocked. J. Exp. Bot. 2017, 68, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T. Cold plasma in food and agriculture. In Plasma in Agriculture. Fundamentals and Applications, 1st ed.; Misra, N.N., Shluter, O., Cullen, P.J., Eds.; Academic Press: London, UK, 2016; pp. 205–222. [Google Scholar]
- Sera, B.; Sery, M. Non-thermal plasma treatment as a new biotechnology in relation to seeds, dry fruits, and grains. Plasma Sci. Technol. 2018, 20, 044012. [Google Scholar] [CrossRef] [Green Version]
- Maffei, M.E. Magnetic field effects on plant growth, development and evolution. Front. Plant Sci. 2014, 5, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrusziewski, S.; Martinez, E. Magnetic field as a method of improving the quality of sowing material: A review. Int. Agrophys. 2015, 29, 377–389. [Google Scholar] [CrossRef]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma agriculture from laboratory to farm: A review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Mildažienė, V.; Paužaitė, G.; Naučienė, Z.; Žūkienė, R.; Malakauskienė, A.; Norkevičienė, E.; Šlepetienė, A.; Stukonis, V.; Olšauskaitė, V.; Padarauskas, A.; et al. Seed treatment with cold plasma and electromagnetic field improves germination, plant growth and induces changes in the amount of major isoflavones in leaves of red clover (Trifolium pratense). J. Phys. D Appl. Phys. 2020, 53, 264001. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hempseed in food industry: Nutritional value, health benefits, and industrial applications. Compr. Rev. Food. Sci. Food Saf. 2019, 19, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Schluttenhofer, C.; Yuan, L. Challenges towards revitalizing hemp: A multifaceted crop. Trends Plant Sci. 2017, 22, 917–929. [Google Scholar] [CrossRef] [Green Version]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sera, B.; Gajdova, I.; Gavril, B.; Hnatiuc, E.; Sery, M.; Spatenka, P. Hemp (Cannabis sativa L.) seeds after plasma treatment. In Proceedings of the 13th International Conference on Optimization of Electrical and Electronic Equipment (OPTIM), Brasov, Romania, 24–26 May 2012; pp. 1371–1374. [Google Scholar]
- Sera, B.; Sery, M.; Gavril, B.I.; Gajdova, I. Seed germination and early growth responses to seed pre-treatment by non-thermal plasma in hemp cultivars (Cannabis sativa L.). Plasma Chem. Plasma Process. 2017, 37, 207–221. [Google Scholar] [CrossRef]
- Iranbakhsh, A.; Oraghi Ardebili, Z.; Molaei, H.; Oraghi Ardebili, N.; Amini, M. Cold plasma up-regulated expressions of WRKY1 transcription factor and genes involved in biosynthesis of cannabinoids in hemp (Cannabis sativa L.). Plasma Chem. Plasma Process. 2020, 40, 527–537. [Google Scholar] [CrossRef]
- Adesina, I.; Bhowmik, A.; Sharma, H.; Shahbazi, A. A review on the current state of knowledge of growing conditions, agronomic soil health practices and utilities of hemp in the United States. Agriculture 2020, 10, 129. [Google Scholar] [CrossRef] [Green Version]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [Green Version]
- Booth, J.K.; Jörg Bohlmann, J. Terpenes in Cannabis sativa—From plant genome to humans. Plant Sci. 2019, 284, 67–72. [Google Scholar] [CrossRef]
- Bußler, S.; Herppich, W.B.; Neugart, S.; Schreiner, M.; Ehlbeck, J.; Rohn, S.; Schlüter, O. Impact of cold atmospheric pressure plasma on physiology and flavonol glycoside profile of peas (Pisum sativum ‘Salamanca’). Food Res. Int. 2015, 76, 132–141. [Google Scholar] [CrossRef]
- Mildažienė, V.; Paužaitė, G.; Naučienė, Z.; Malakauskiene, A.; Žūkienė, R.; Januškaitienė, I.; Jakštas, V.; Ivanauskas, L.; Filatova, I.; Lyushkevich, V. Pre-sowing seed treatment with cold plasma and electromagnetic field increases secondary metabolite content in purple coneflower (Echinacea purpurea) leaves. Plasma Process. Polym. 2018, 14, 1700059. [Google Scholar] [CrossRef]
- Mildažienė, V.; Ivankov, A.; Paužaitė, G.; Naučienė, Z.; Žūkienė, R.; Degutytė-Fomins, L.; Pukalskas, A.; Venskutonis, P.R.; Filatova, I.; Lyushkevich, V. Seed treatment with cold plasma and electromagnetic field induces changes in red clover root growth dynamics, flavonoid exudation, and activates nodulation. Plasma Process. Polym. 2020, 17, 2000160. [Google Scholar]
- Mildaziene, V.; Pauzaite, G.; Malakauskiene, A.; Zukiene, R.; Nauciene, Z.; Filatova, I.; Azharonok, V.; Lyushkevich, V. Response of perennial woody plants to seed treatment by electromagnetic field and low-temperature plasma. Bioelectromagnetics 2016, 37, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Filatova, I.; Lyushkevich, V.; Goncharik, S.; Zhukovsky, A.; Krupenko, N.; Kalatskaja, J. The effect of low-pressure plasma treatment of seeds on the plant resistance to pathogens and crop yields. J. Phys. D Appl. Phys. 2020, 53, 244001. [Google Scholar] [CrossRef]
- Richards, F.J. A flexible growth function for empirical use. J. Exp. Bot. 1959, 10, 290–300. [Google Scholar] [CrossRef]
- Hara, Y. Calculation of population parameters using Richards function and application of indices of growth and seed vigor to rice plants. Plant Prod. Sci. 1999, 2, 129–135. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Yin, M.Q.; Huang, M.J.; Ma, B.Z.; Ma, T.C. Stimulating effects of seed treatment by magnetized plasma on tomato growth and yield. Plasma Sci. Technol. 2005, 7, 3143–3147. [Google Scholar]
- Zhou, Z.; Huang, Y.; Yang, S.; Chen, W. Introduction of a new atmospheric pressure plasma device and application on tomato seeds. Agricult. Sci. 2011, 2, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Lu, Y.; Li, J.; Li, L.; He, X.; Shao, H.; Dong, S. Effect of cold plasma treatment on seed germination and growth of wheat. PLoS ONE 2014, 9, e97753. [Google Scholar] [CrossRef] [Green Version]
- Paužaitė, G.; Malakauskiene, A.; Naučienė, Z.; Žūkienė, R.; Filatova, I.; Lyushkevich, V.; Azarko, I.; Mildažienė, V. Changes in Norway spruce germination and growth induced by pre-sowing seed treatment with cold plasma and electromagnetic field: Short-term versus long-term effects. Plasma Process. Polym. 2018, 14, 1700068. [Google Scholar] [CrossRef]
- Podlesny, J.; Misiak, L.E.; Podlesna, A.; Pietruszewski, S. Concentration of free radicals in pea seeds after pre-sowing treatment with magnetic field. Int. Agrophys. 2005, 19, 243–249. [Google Scholar]
- Azharonok, V.; Goncharik, S.V.; Filatova, I.I.; Shik, A.S.; Antonyuk, A.S. The effect of the high frequency electromagnetic treatment of the sowing material for legumes on their sowing quality and productivity. Surf. Eng. Appl. Electrochem. 2009, 45, 317–327. [Google Scholar] [CrossRef]
- Bilalis, D.J.; Katsenios, N.; Efthimiadou, A.; Karkanis, A. Pulsed electromagnetic field: An organic compatible method to promote plant growth and yield in two corn types. Electromagn. Biol. Med. 2012, 31, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Bautista, R.; Hernandez-Aguilar, C.; Suazo-Lopez, F.; Dominguez-Pachecco, A.F.; Virgen-Vargas, J.; Perez-Reyes, C.; Peon-Escalante, I. Electromagnetic field in corn grain production and health. Afr. J. Biotechnol. 2014, 13, 76–83. [Google Scholar]
- Pavlovic, R.; Panseri, S.; Giupponi, L.; Leoni, V.; Citti, C.; Cattaneo, C.; Cavaletto, M.; Giorgi, A. Phytochemical and ecological analysis of two varieties of hemp (Cannabis sativa L.) grown in a mountain environment of Italian Alps. Front. Plant Sci. 2019, 10, 1265. [Google Scholar] [CrossRef] [PubMed]
- Taoufik, B.; Hamid, S.; Aziz, E.; Abdellah, F.; Seddik, S.; Yassine, E.; Mouhcine, F.; Zoubida, C.; Mohame, T. Comparative study of three varieties of Cannabis sativa L. cultivate in different region of Morocco. Int. J. Pharm. Phytochem. Res. 2017, 9, 643–653. [Google Scholar] [CrossRef] [Green Version]
- Zukiene, R.; Nauciene, Z.; Januskaitiene, I.; Pauzaite, G.; Mildaziene, V.; Koga, K.; Shiratani, M. DBD plasma treatment induced changes in sunflower seed germination, phytohormone balance, and seedling growth. Appl. Phys. Express 2019, 12, 126003. [Google Scholar] [CrossRef]
- Whitehead, C.J. The Chemistry of Cold Plasma. In Plasma in Agriculture. Fundamentals and Applications, 1st ed.; Misra, N.N., Shluter, O., Cullen, P.J., Eds.; Academic Press: London, UK, 2016; pp. 53–82. [Google Scholar]
- Considine, M.J.; Diaz-Vivancos, P.; Kerchev, P.; Signorelli, S.; Agudelo-Romero, P.; Gibbs, D.J.; Foyer, C.H. Learning to breathe: Developmental phase transitions in oxygen status. Trends Plant Sci. 2017, 22, 140–153. [Google Scholar] [CrossRef] [Green Version]
- Richards, S.L.; Wilkins, K.A.; Swarbreck, S.M.; Anderson, A.A.; Habib, N.; Smith, A.G.; McAinsh, A.; Davies, J.M. The hydroxyl radical in plants: From seed to seed. J. Exp. Bot. 2014, 66, 37–46. [Google Scholar] [CrossRef]
- Bailly, C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci. Rep. 2012, 2, 741. [Google Scholar] [CrossRef]
- Ling, L.; Jiafeng, J.; Jiangang, L.; Minchong, S.; Xin, H.; Hanliang, S.; Yuanhua, D. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Groot, G.J.J.A.; Hundt, A.; Murphy, A.B.; Bange, M.P.; Mai-Prochnow, A. Cold plasma treatment for cotton seed germination improvement. Sci. Rep. 2018, 8, 14372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Ramírez, A.; López-Santos, C.; Cantos, M.; García, J.L.; Molina, R.; Cotrino, J.; Espinós, J.P.; González-Elipe, A.R. Surface chemistry and germination improvement of Quinoa seeds subjected to plasma activation. Sci. Rep. 2017, 7, 5924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Los, A.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Bourke, P. Investigation of mechanisms involved in germination enhancement of wheat (Triticum aestivum) by cold plasma: Effects on seed surface chemistry and characteristics. Plasma Process. Polym. 2019, 16, 1800184. [Google Scholar] [CrossRef]
- Koga, K.; Attri, P.; Kamataki, K.; Itakagi, N.; Shiratani, M.; Mildažienė, V. Impact of radish sprouts seeds coat color on the electron paramagnetic resonance signals after plasma treatment. Jpn. J. Appl. Phys. 2020, 59, SHHF01. [Google Scholar] [CrossRef]
- Degutytė-Fomins, L.; Paužaitė, G.; Žukienė, R.; Mildažienė, V.; Koga, K.; Shiratani, M. Relationship between cold plasma treatment-induced changes in radish seed germination and phytohormone balance. Jpn. J. Appl. Phys. 2020, 59, SH1001. [Google Scholar] [CrossRef]
- Mildažienė, V.; Aleknavičiūtė, V.; Žūkienė, R.; Paužaitė, G.; Naučienė, Z.; Filatova, I.; Lyushkevich, V.; Haimi, P.; Tamošiūnė, I.; Baniulis, D. Treatment of common sunflower (Helianthus annus L.) seeds with radio-frequency electromagnetic field and cold plasma induces changes in seed phytohormone balance, seedling development and leaf protein expression. Sci. Rep. 2019, 9, 6437. [Google Scholar] [CrossRef]
- Horkay, E.; Bócsa, I. Objective basis for the evolution of differences in fibre quality between male, female and monoecious hemp plants. J. Internat. Hemp Assoc. 1996, 3, 67–68. [Google Scholar]






| Treatment | Vi (%) | Me (Hours) | Qu (Hours) |
|---|---|---|---|
| Control | 58.9 ± 4.8 | 64.1 ± 1.1 | 8.6 ± 2.7 |
| Vacuum | 56.7 ± 7.7 | 68.7 ± 3.8 | 8.6 ± 2.1 |
| CP5 | 78.9 ± 4.0 * | 50.8 ± 2.2 * | 9.6 ± 3.1 |
| EMF15 | 86.7 ± 3.8 * | 61.7 ± 3.4 | 9.0 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivankov, A.; Nauciene, Z.; Zukiene, R.; Degutyte-Fomins, L.; Malakauskiene, A.; Kraujalis, P.; Venskutonis, P.R.; Filatova, I.; Lyushkevich, V.; Mildaziene, V. Changes in Growth and Production of Non-Psychotropic Cannabinoids Induced by Pre-Sowing Treatment of Hemp Seeds with Cold Plasma, Vacuum and Electromagnetic Field. Appl. Sci. 2020, 10, 8519. https://doi.org/10.3390/app10238519
Ivankov A, Nauciene Z, Zukiene R, Degutyte-Fomins L, Malakauskiene A, Kraujalis P, Venskutonis PR, Filatova I, Lyushkevich V, Mildaziene V. Changes in Growth and Production of Non-Psychotropic Cannabinoids Induced by Pre-Sowing Treatment of Hemp Seeds with Cold Plasma, Vacuum and Electromagnetic Field. Applied Sciences. 2020; 10(23):8519. https://doi.org/10.3390/app10238519
Chicago/Turabian StyleIvankov, Anatolii, Zita Nauciene, Rasa Zukiene, Laima Degutyte-Fomins, Asta Malakauskiene, Paulius Kraujalis, Petras Rimantas Venskutonis, Irina Filatova, Veronika Lyushkevich, and Vida Mildaziene. 2020. "Changes in Growth and Production of Non-Psychotropic Cannabinoids Induced by Pre-Sowing Treatment of Hemp Seeds with Cold Plasma, Vacuum and Electromagnetic Field" Applied Sciences 10, no. 23: 8519. https://doi.org/10.3390/app10238519
APA StyleIvankov, A., Nauciene, Z., Zukiene, R., Degutyte-Fomins, L., Malakauskiene, A., Kraujalis, P., Venskutonis, P. R., Filatova, I., Lyushkevich, V., & Mildaziene, V. (2020). Changes in Growth and Production of Non-Psychotropic Cannabinoids Induced by Pre-Sowing Treatment of Hemp Seeds with Cold Plasma, Vacuum and Electromagnetic Field. Applied Sciences, 10(23), 8519. https://doi.org/10.3390/app10238519