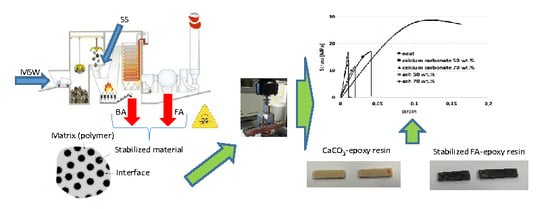
1. Introduction
Incineration is an effective method to manage municipal solid wastes (MSW). Indeed, the waste volume is reduced up to 90%, with the by-product to concentrate persistent pollutants in combustion residues. According to European Union waste management strategy, the use of bottom ash (BA) is generally suggested as a sustainable solution to manage this secondary waste [
1]. Indeed, MSW incineration BA is ever more identified as a potential source for materials of great importance to the economy [
2]. BA was been proposed to be reused, for example, in building industry [
3]. Incorporation of BA has already been demonstrated by Nikravan et al. for the partial substitution of aggregate and filler in the case of concrete construction [
4]. In addition, BA reuse as adsorbents has been investigated, due to its high porosity and large surface area [
5].
On the contrary, fly ash (FA), which is the thinnest fraction of the particulate contained in combustion flue gas [
6], is considered a hazardous waste because of the presence of leachable heavy metals such as Cd, Hg, Pb and Zn and organic micro-pollutants (polychlorinated dibenzodioxins, PCDD and polychlorinated dibenzofurans, PCDF).
Generally, cement stabilized FA is landfilled, due to dangerous effects on environment and humans associated with a direct disposal. On the other hand, Europe aims to develop a circle economy strategy, there is an increasing need to encourage FA stabilization and recovery [
7].
Several technologies, such as cement based techniques, chemical stabilization treatments, washing [
8] and thermal techniques have been proposed to treat FA [
9]. In particular, in recent years, the idea to develop sustainable technologies for waste remediation, in the frame of Azure chemistry approach [
10], has contributed to promote the use of by-products and waste materials, as stabilizing agents, instead of use natural resources [
11]. For example, the COSMOS technology [
12,
13,
14], initially based on the use of colloidal silica to stabilize leachable heavy metals (contained in FA), was improved by the replacement of commercial silica source with by-products, such as rice husk ash [
15,
16], silica fume [
17] and sewage sludge ash [
18]. Very recently, it was also shown that this kind of treatment allows us to sequestrate carbon dioxide [
19], with increased sustainability of the proposed approach [
20].
Finally, FA was treated by using BA, considered as a new urban mining source due to the pozzolanic characteristics of BA [
21]. This can be really considered a zero-waste treatment, requiring only mixing of ashes deriving from the same incinerator. This technology seems to make a sustainable process, that can be directly applied on the thermal treatment plant.
In the past, it was shown that COSMOS technologies allow the obtaining of a filler, with different possible applications [
22], that is, in plastic composites [
23,
24,
25].
In the present paper, the filler obtained by using BA to stabilize FA was considered for the production of epoxy resin matrix composite materials. The aim of this work is to characterize and evaluate the real possibility of the new filler reuse. In addition, an evaluation of the advantages, in terms of energies and emissions required for the material synthesis are quantified with respect to the use other resources. For this aim, a recently proposed simplified approach is used [
7].
This work can be considered a virtuous example of resource use efficiency, with focus on wastes—the circular economy approach assumes that the resources extracted and produced must be always in circulation, avoiding the landfilling. In this specific case, the reuse of stabilized waste is proposed in order to promote the transition to a more circular economy (CE), by eco-innovation aiming at a ‘‘zero waste economy.” And even though the European Union (EU) action plan about CE does not explicitly address ashes from waste incineration, using these resources more efficiently may contribute to reach some scopes defined in the CE action plan and helps to close a gap in the CE loop [
26].
2. Materials and Methods
In this study, Coal Fly Ash (CFA), Flue Gas Desulphurization (FGD), FA and BA were considered. CFA is produced as a by-product in thermal power plant during coal combustion. Fly ashes, classified as fine particulate residues, were removed from the combustion gases before they are emitted into the atmosphere by a dust-collection system [
27]. FGD residues are another by-product resulting from coal combustion processes in power plants. In this process, insoluble calcium sulphite and calcium sulphate solids are formed due to the use of a lime-based or a sodium-based reagent [
28].
In the present work, CFA and FGD residues were collected at the pulverized coal thermal power plant located in Brescia, Italy. BA and FA were provided by the Brescia (Italy) municipal solid waste incinerator plant. The latter works with three combustion lines per day, with a capacity of 880 tons MSW ashes each. For years, co-combustion of municipal solid waste with sewage sludge has been applied at this incineration plant. Large particles were separated from BA before their use. Afterwards, a milling procedure was applied to BA portion in order to obtain particles less than 700 µm.
The flue gas cleaning system involves the uses of dry scrubbers, fabric filters, an activated carbon injection system and a combination selective non-catalytic and catalytic reductions. Moreover, the plant contains a device installed to monitor the continuous flue gas emissions.
An industrial grade calcium carbonate (CaCO3) Calcite V40 (Carrara, Italy), with a particle diameter distribution below 60 µm (99%), was used as a reference to produce polymer filler composites. The composite material matrix consists of Epoxy resin E-227 (produced by Prochima), also known as polyepoxides. This is a class of reactive pre-polymers and polymers characterized by the presence of epoxide groups. The bi-component product is formed by polymer and catalyst that must be mixed according to the ratio indicated by the manufacture equal to 1/2 in weight to obtain the final product.
2.1. Stabilization Procedure on the Pilot Plant
FA was stabilized by using the pilot plant (see
Figure 1) placed in a Brescia incinerator, as described by Benassi et al. [
13]. FGD and CFA were mixed with FA with relative weight percentage fixed at 65% FA, 20% FGD and 15% CFA. Calcium sulfite hydrate and portlandite are identified as the main crystalline phases of FGD residues. CFA revealed only two crystalline phases:—quartz and mullite [
29]. In this study, the sample was prepared by mixing FA, CFA, FGD and BA, as reported by Assi et al. [
21]. Tap water (25 kg) at room temperature was also used. The mixing procedure was made for 30 min as reported by Benassi et al. [
13]. After the synthesis, the material underwent a natural aging process at the incinerator plant for 11 months, in order to promote carbonation reactions, that contribute to the stabilization of heavy metals over time as already discussed by Assi et al. [
19]. The sample stability was verified by performing leaching tests on the stabilized product at 1, 2, 3 and 11 months after the treatment.
2.2. Preparation of Composite Samples
Calcite and stabilized sample were used as filler. A series of five specimens:—0%, 10%, 30%, 50% and 70% wt were prepared by mixing the epoxy resin with calcite or stabilized sample in a beaker for about 5 min. After that, the mixture was drained in a mold and maintained for 24 h at room temperature to obtain a sufficient hardness. The samples of epoxy composites with different filler were fabricated according to the following steps:—(i) silicone rubber molds have been produced in order to realize the negative shape of the samples;—(ii) the stabilized sample has been mixed with the epoxy resin before pouring the mix in the molds; (iii) material stand-by for 4 days at room temperature. The same procedure has been applied to prepare the specimens with calcite as a filler.
Figure 2 shows the two different samples prepared with stabilized FA and calcite, respectively.
2.3. Characterization
2.3.1. Leaching Tests
Leaching test was performed to raw powders and stabilized sample according the CEN normative (CEN EN 12457-2) [
30] and optimized procedure [
14,
21]. For leaching tests, 20 g of each sample were mixed with 200 mL MQ water (1:10) and mixed at room temperature for 2 h by a magnetic stirrer. After that, samples were filtered through 0.45 µm pore membranes and the pH of the filtrates was checked by pH-meter (Metrohm, model 827 Lab, Origgio, Italy). The analyses of leaching solutions were scheduled at one (1 M), two (2 M), three (3 M) and eleven months (11 M) after the stabilization, in order to investigate the efficacy of the process.
Elemental chemical analysis of the leachate solutions was performed by means of Total reflection X-Ray Fluorescence (TXRF) technique. The S2 Picofox system from Bruker (Bruker AXS Microanalysis GmbH, Berlin, Germany), equipped with a Mo tube operating at 50 kV and 750 µA and a Silicon Drift Detector (SDD), was employed. Internal standard method is used to calculate the selected analytes concentration. To this aim, a stock solution of 1 g/L Ga in nitric acid (Ga-ICP Standard Solution, Fluka, Sigma Aldrich, Saint Louis, MO, USA) was used. A solution with final concentration of 1 mg/L of Ga was obtained by adding about 10 µL of a solution of 100 mg/L of Ga to 1 mL solution. In order to obtain homogenized working solutions, a vortex shaker at 2500 rpm was employed for 1 min. A droplet of 10 µL was deposited onto the surface of an acrylic reflector in a central position. Before measurements, the reflectors were left dried under a laminar hood on a hot plate at 50 °C and the residues were measured. For each sample, three independent reflectors were prepared and irradiated for 600 s of live time. All the recorded TXRF spectra were analyzed with the instrumental software. In these experimental conditions, P, S and Cl are low Z elements such as P, S and Cl that are underestimated [
31]. For this reason, the quantification of these elements was corrected using the calculated relative sensitivities for P and S obtained by the calibration curves [
32].
2.3.2. XRD
X-ray diffraction (XRD) is performed for structural and microstructural characterization of the raw powders [
21] and the stabilized sample after 11 M (see
Figure 3). XRD patterns were acquired by means of an X’Pert PRO Bragg Brentano diffractometer (Malvern PANalytical, UK) equipped with a Cu K anode and operating at 40 kV and a current of 40 mA. Scans (2θ) were performed from 5° to 90°, with a step interval of 0.0167°. Philips X’Pert software (associated with the COD open database) was employed for phase identification.
2.3.3. Three Point Bending Test
Three point bending tests were performed by means of an electromechanical dynamometer (Instron Mod 3366 Tensile Tester, Akribis Scientific Limited, Knutsford, UK) equipped with a 10 kN load cell. The tests were conducted at a crosshead speed of 5 mm/min and the support span of 64 mm at room temperature. Measuring 80 mm × 4 mm × 10 mm, five samples of each specification are taken. The flexural strength was calculated with results being calculated with the following Equation (1) (EN ISO 178:2010) [
33]:
where
σfmax is the flexural strength (MPa),
P is the load (N),
L is the support span (mm),
b is the sample width (mm) and
d is the sample thickness (mm).
2.3.4. SEM-EDXS
The morphology of the raw powders and obtained samples was characterized by a scanning electron microscope (SEM) Zeiss LEO EVO 40XVP (Carl Zeiss Microscopy GmbH, Oberkochen, Germany) equipped with a Link Analytical probe for energy dispersive X-ray spectroscopy (EDXS, Oxford Instruments NanoAnalysis, Buckinghamshire, UK). Gold was deposited on the sample surface in order to ensure the electrical charge. Secondary electron mode was employed to characterize the composites the morphology.
2.4. Evaluation of the Sustainability of the Proposed Technology
Recently, a rapid and suitable method to compare two materials, based on an index, was been introduced [
7,
34]. This approach is named ESACPE, standing for Evaluation of Sustainability of material substitution using carbon footprint by a simplified approach. This approach was proposed to support industrial transition to CE, by means of a method, based on the use of simplified indicators, for the evaluation of raw materials substitution sustainability.
An index (SUB-RAW index, see in the following) was introduced as a parameter to quantify the environmental benefices of a raw material substitution. It is evaluated only taking into account two parameters, strictly connected to the environmental performance of the considered materials, that are embodied energy (EE) and CO2 footprint (CF).
The materials production from ores and feedstock requires energy, which is defined as “embodied energy.” EE accounts for all direct and indirect energies needed for the production of 1 kg of a material [
35]. The CO
2 footprint corresponds to the equivalent mass of greenhouse gases (kg CO
2 equivalent), produced and released into the atmosphere due to the production of 1 kg of the material [
35]. In general, The EE and CF account for the resources and the emissions, respectively, involved in the synthesis of a material.
These parameters do not take into account functional and economical properties of the considered materials. Indeed, the basic idea of the proposed method is to use a simplified approach to evaluate material substitution. This is the reason the procedure must be simple and accessible to all.
Eventually after the positive results obtained by the ESCAPE method it would be possible to perform complete Life Cycle Assessment analysis, to globally evaluate the proposed material substitution.
The SUB-RAW index is defined as:
where the subscripts
raw and
sub stand for raw material and new proposed material, respectively.
The EE and CF range from 0.01 to 106–107, respectively reported in kg/kg and MJ/kg. Due to the large range of values, a logarithmic scale is often used. All the EE and CF parameters are used without their dimension, obtaining a dimensionless numerical value. It corresponds to a mean parameter accounting for environmental pressure and energies of the two materials, that are compared.
The goal to substitute natural resources with more sustainable materials is obtained when the index results positive. A positive value of this index, even if it is very low, can be a very good result in terms of substitution.
For example, a SUB-RAW index of about 0, 1, indeed, corresponds to a mean variation of EE and CF of about 25%.
3. Results and Discussion
3.1. Samples Characterization
3.1.1. TXRF Analysis
TXRF results of leachate solutions of raw ashes are reported in
Table 1, with good agreement with previous studies [
36,
37,
38]. The major elements in leachable FA are Cl, Ca and Br. Ca is also identified as the essential constituent of BA but its higher presence in FA is expected due to the addition of Ca(OH)
2 to adsorb acid gases (like SO
2 and HCl), produced during the incineration process. S is another element found in higher amount in FA with respect to BA due to the lower boiling points and higher volatility of the sulphate phases [
38]. Other elements are found in trace amounts in all raw ashes. Zn and Pb show significant differences between FA and BA, in agreement with reported literature [
13,
38]. These two heavy metals are almost one order of magnitude higher in FA compared to BA and lower in CFA and FGD. As reported in
Table 1, these ashes contain mostly Ca and S [
14,
39], while other elements are in low concentrations. Literature reports extremely high concentration of Si in CFA [
27] but under this experimental conditions, Si could not be quantified.
Stabilized sample revealed a noticeable decrease in Pb and Zn leaching after the first month with respect to the raw FA. While, after 2 M, Pb concentration is lower than lower detection limit (LOD) by TXRF spectrometry (see
Table 2), which is 0.002 mg/L [
40]. In contrast, stabilized sample revealed higher S concentration compared with FA due to FGD residues addition for stabilization. Based on data reported in
Table 2, we can conclude that the lower Zn and Pb leachability must be attributed to the carbonation and pozzolanic reactions that occur during stabilization [
21]. It is already shown that pH is a key factor that affects heavy metals leaching behaviors. Considering the pH of all raw powders and final stabilized mixtures, it is extremely interesting to note that all ashes have a starting pH higher than 12 (see
Table 1), while stabilized sample has a pH lower than 11 (see
Table 2). In this way, pH monitoring also allows the following of the carbonation reactions [
19].
In particular, the natural aging of stabilized material allows us to sequestrate carbon dioxide, making it possible to take advantage of the carbonation reaction, as a by-product of the stabilization mechanism [
19]. Indeed, carbonation produces a pH neutralizing effect (the pH evolution of stabilized material, with time is reported in
Table 2, which contributes to decrease the solubility/mobility of heavy metals.
3.1.2. XRD Pattern
Figure 3 reports the XRD pattern of the stabilized material 11 months after the synthesis procedure. The following crystalline phases are detected:—halite (NaCl), calcite (CaCO
3), quartz (SiO
2), hannebachite (Ca
2H
2O
7S
2), belite (Ca
2SiO
5), sylvine (KCl) and bassanite (Ca
3H
4O
13.8S
3).
The material is made of the same crystalline phases already detected in similar materials, stabilized by using amorphous silica sources [
11,
18], which are analyzed weeks after the treatment. The XRD spectra collected on stabilized material 1 month after the material synthesis (data not shown) corresponds to the one already reported by Assi et al. [
21], due to the same stabilization procedure adopted. However, it is worth to notice the presence of belite in this sample. This phase was not found in previous works using the proposed stabilization methods [
41] and is generally present as an additional main mineralogical component of calcium sulfoaluminate cement, which can be obtained by using FA [
42]. Then its presence, although it was not expected, can be justified by previous works. It was already shown that FA and a source of lime (derived from FGD residues introduced in the sample stabilization procedure) respectively served as the principal raw silica and lime sources during the production of building materials such as clinker [
43]. In the present case, the addition of BA allows us to increase the amount of CaO-Al
2O
3-SiO
2 able to increase the reactive amorphous phase that contribute to the heavy metals stabilization [
44]. The belite formation is probably due to the crystallization of C-S-H (calcium silicate hydrate) phase, due to the reaction between silicate species and calcium, which in cement is a hydration product.
The stabilized material contains ceramic and silica-based material. This allows us to suppose that it may be reused to substitute some natural resources, with similar composition. One advantage of the obtained stabilized material is its physical form. Being a powder, it may be used as a filler in a composite, realized by mixing a matrix with a powder. In principle, this material may be used with all matrices requiring inorganic filler. In the present work, this filler was used to produce some composites by using an epoxy resin E-227, as described in the experimental section.
3.1.3. Three Point Bending Test
Flexural strength and modulus of elasticity were obtained through a three-point bending test.
Figure 4 reports a stress-strain curves obtained at different content (50% and 70%) of calcium carbonate and stabilized sample (ash) in comparison with neat polymer. Flexural modulus, evaluated from the slope of the stress versus strain deflection curve determines how much a sample will bend when a given load is applied, while flexural strength is calculated as the maximum bending stress.
In
Figure 4, it is possible to notice first that the neat epoxy shows an elongation at break much higher than that both stabilized sample-epoxy and calcium carbonate-epoxy composites. Moreover, the neat epoxy did not break brittle but it yields above the peak. The presence of 50% by weight of filler significantly reduces the elongation at break of the composites and a major amount of filler up to 70% by weight further decreases it. The influence of stabilized FA as filler in epoxy resin matrix on the elongation at break is similar to that of calcium carbonate. As expected, increasing the filler amount in the epoxy matrix the elongation and the straight at break decrease, while the flexural modulus increases [
45,
46,
47]. In general, the same content [% wt] of ash and calcium carbonate affects the composite flexural behavior similarly. The flexural modulus and flexural strength of CaCO
3/epoxy and stabilized sample/epoxy composites with different filler amount are shown in
Figure 5 and
Figure 6, respectively.
Figure 5 shows the trend of flexural modulus of stabilized sample-epoxy and calcium carbonate-epoxy composites at different filler content (from 10% up to 70% by weight). It should be noted that the flexural modulus of stabilized sample (ash) and calcium carbonate composites is similar for each filler content and the curves almost overlap. The flexural modulus remains approximately constant up to a filler amount of 30% wt, then it slightly increases to a filler amount of 50% and it strongly increases for filler amount of 70%wt. In general, the flexural modulus raises increasing the filler content by more than 30% by weight of filler content independently of whether the filler is stabilized sample or calcium carbonate.
Figure 6 shows the trend of flexural strength for of stabilized sample-epoxy and calcium carbonate-epoxy composites at different filler content (from 10% up to 70% by weight). Flexural strength and elongation at brake reduction of composites decreased with increasing of filler loading mainly due to agglomeration process acting as defects in the polymer matrix. It is worthwhile to note that the flexural strength decreases increasing the filler content up to 30% by weight for both ash and calcium carbonate. The composite stabilized sample-epoxy shows a constant decrease in the flexural strength up to a filler content of 50% wt and then it shows a slight increase up to 70% wt. Due to this trend and its standard deviation, it is possible to consider the flexural strength constant for stabilized sample content above 30% wt as well as for calcium carbonate composite. The experimental data show a change in tendency of flexural modulus and strength for stabilized FA and calcium carbonate content greater than 30% by weight.
3.1.4. SEM-EDXS Analysis
SEM–EDXS was performed on the longitudinal surface of the specimens, that were in contact with the mold.
Figure 7 presents SEM images of 50% wt stabilized sample filled epoxy composite in comparison with 50% wt CaCO
3 filled epoxy composite. SEM images demonstrate a significant difference between stabilized sample and CaCO
3 based composite. Indeed, the composite material with stabilized sample filler exhibits a non-homogeneous dispersion of particles with different dimensions, characterized by large agglomerates. These results are confirmed by the EDX maps (see
Figure 8) that show the distribution of Ca in different % wt filler. On the other hand, a homogeneous distribution is associated with a better dispersion, with particles with diameter below 60 μm observed for composite with CaCO
3. Ca was chosen because is present in both fillers and its quantity is limited in the epoxy-resin matrix. This allows us to highlight that particle agglomeration takes place in the case of the new fillers composite. Besides, particle cluster occur above 10% wt of stabilized sample, thus leading to a larger particle size and hence, strengthening decrease. When particles aggregation occurred, due to the interactions between them within the polymer matrix, the particle size increased and led to the creation of stress concentration points, which act as crack initiation and eventually weakened the interfacial adhesion between the matrix and particle [
46]. The addition of stabilized sample fillers to the composite material leads to the poor distribution of particles in the resin matrix [
47].
From the elemental characterization provided by different chemical EDXS analyses (
Table 3), the increase of filler amount causes a decrease in the C and Cl content, while increases Ca amount. In case of composite with stabilized sample, Al, Mg, K and S presence has been noticed, due to the initial chemical composition of the FA. It is important to highlight the limit of the instrument with light elements such as C; however, although the C amount is expressed in a semi-quantitative way, it becomes very useful for the comparative analysis between the two examined compounds.
3.2. Sustainability Evaluation of the Proposed Technology
EE and CF parameters of the synthesis of the proposed new filler were considered to evaluate environmental sustainability.
Data about EE and CF of the recycled stabilized material were obtained by using the CES Selector software [
48] taking into account the stabilization procedure, which involves the bottom ash grinding and considering the mixing of different ashes typologies, as described in the experimental section and discussed in literature [
21]. For the stabilized material, an embodied energy ranging from 0.061 to 0.062 MJ/kg and a CO
2 footprint of about 0.03 kg/kg resulted.
Table 4 reports the SUB-RAW indices evaluated by comparing the new proposed material with respect to calcite, which is a natural resource that is often used as a filler, to produce some composites. As a reference, this index was also calculated in comparison with sandstone, talc and resin used to synthesize the composites for this work.
From calculated SUB-RAW indices, it is evident that the new proposed inert is more sustainable if compared to some natural resources, as for example calcite, talc and sandstone. Obviously it is also more sustainable with respect to a resin [
49]. Then, it is possible to conclude that it may be used to produce composited materials. In principle, its market applicability is potentially very promising. To evaluate this, also the price of the materials reported in this work have been considered.
Figure 9 shows the EE for several materials (ceramic, glass and composites) as a function of their commercial price. Data were obtained from CES Selector 2019 [
48]. Data on the carbon footprint of the same materials, reported as a function of their commercial price, have the same behavior already shown by Bontempi et al. [
34], therefore they are not reported in this paper. In correspondence of the EE of the new proposed material, an orange dotted horizontal line is reported.
From the analysis of
Figure 9, it can be seen that the new obtained stabilized material can be compared to wollastonite in terms of the EE environmental parameter used to quantify the sustainability of a material.
Figure 9 shows that the energy needed to synthesize the new proposed material is lower than that of the almost majority of ceramic and glass materials. It results evident that it is more sustainable that the previous proposed COSMOS filler, obtained by adding an external silica source [
16,
39] to stabilize MSWI FA. Indeed, EE of COSMOS resulted higher than that of calcite. On the contrary, in the present case, it is demonstrated that the new obtained stabilized material can be used, with environmental advantages, in substitution of calcite. This is due to the fact that BA is a by-product produced on the same incinerator plant where FA is generated. As a consequence, its EE and CF can be assumed to be zero. It is possible to conclude that the new filler is strongly suggested to be used for the synthesis of composites materials, avoiding the natural resources employ. The price, which is generally used to evaluate the commercial applications of a material, is an important criterion, which must be also taken into account, because it can be related to the new material market. In the present case, this is unknown but the
Figure 9 reports the price of several materials, allowing us to verify the market opportunities for the new proposed filler.
4. Conclusions
Recently, a new stabilization technology developed to treat MSW incineration FA has been proposed. It is based on the use of bottom ash, produced at the same incinerator plant. This new process is considered a zero-waste approach, because it involves the use of waste materials produced on the same plant. As a consequence, the landfilling of these wastes can be neglected, following the recommended material use close loop by utilizing ‘waste’ for suitable and sustainable applications.
This work demonstrates the sustainability of the proposed technology by using a new simplified approach and allowing to compare the obtained inert material with natural resources.
In addition, the effects of the new produced filler on the structure and properties of obtained composites based on the use of a resin are assessed by means of structural and mechanical analyses. For comparison purposes, also calcite (CaCO3) was used as a standard filler, to formulate composites that were produced and analyzed under the same conditions.
Regarding mechanical properties of the new stabilized sample- composite, the comparison with CaCO3 based formulation shows comparable values in terms of flexural modulus and flexural strength that raise by increasing the filler content around 30% wt, independently of filler type, stabilized sample or calcium carbonate, respectively. The amount of CaCO3 or stabilized sample in the composites is the main factor causing a reduction in the mechanical properties mainly due to the crowding effect. EDX maps highlighted a more homogeneous distribution of Ca in CaCO3-composites with respect to stabilized sample-composites, due to the lower particle dimension in calcite powder.
In summary, it is possible to conclude that the new proposed filler, realized by using stabilized MSWI fly ash, can be used to produce sustainable composites, allowing to preserve natural resources, as for example of calcite, demonstrating similar mechanical and structural performance and with great environmental and economic benefits.
If Europe-wide implementation of waste recovery is to be part of the circular economy strategy, it is expected that one of the political priorities should be the implementation of a legal framework that can support new technologies and materials availability for the recycling sectors.
5. Patents
Patent number 102019000006651, 2019.
Author Contributions
Conceptualization, E.B. and L.E.D.; Methodology, E.B., A.A., F.B.; Software: A.A., G.R., A.G.; Investigation, A.A., F.B., A.Z. (Alessandra Zanoletti), S.D., A.Z. (Annalisa Zacco); Data curation, G.R., A.G., A.Z. (Annalisa Zacco); Writing—original draft preparation, E.B., A.A. and F.B.; Writing—review and editing, E.B., G.R., S.F., L.E.D.; Supervision, E.B., F.B.; Project administration, E.B.; Funding acquisition, E.B., please turn to the CRediT taxonomy for the term explanation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministero dell’Ambiente e della Tutela del Territorio e del Mare and supported by Regione Lombardia, INSTM, CSMT and University of Brescia, project: Energy recovery of waste sludge and their re-use as an alternative to some natural resources, for the production of “Green” composites, RENDERING, CUP: D71I18000170008.
Acknowledgments
The authors acknowledge Giovanna Cornacchia for the support of SEM images and insights.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brunner, P.H.; Rechberger, H. Waste to energy—Key element for sustainable waste management. Waste Manag. 2015, 37, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Boesch, M.E.; Vadenbo, C.; Saner, D.; Huter, C.; Hellweg, S. An LCA model for waste incineration enhanced with new technologies for metal recovery and application to the case of Switzerland. Waste Manag. 2014, 34, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Saikia, N.; Kato, S.; Kojima, T. Production of cement clinkers from municipal solid waste incineration (MSWI) fly ash. Waste Manag. 2007, 27, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Nikravan, M.; Ramezanianpnour, A.A.; Maknoon, R. Technological and environmental behavior of petrochemical incineration bottom ash (PI-BA) in cement-based using nano-SiO2 and silica fume (SF). Constr. Build. Mater. 2018, 191, 1042–1052. [Google Scholar] [CrossRef]
- Chaspoul, F.R.; Le Droguene, M.F.; Barban, G.; Rose, J.C.; Gallice, P.M. A role for adsorption in lead leachability from MSWI bottom ASH. Waste Manag. 2008, 28, 1324–1330. [Google Scholar] [CrossRef]
- Zacco, A.; Borgese, L.; Gianoncelli, A.; Struis, R.P.W.J.; Depero, L.E.; Bontempi, E. Review of fly ash inertisation treatments and recycling. Environ. Chem. Lett. 2014, 12, 153–175. [Google Scholar] [CrossRef]
- Bontempi, E. Raw Materials Substitution Sustainability; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-60830-3. [Google Scholar]
- Fabbricino, M.; Ferraro, A.; Luongo, V.; Pontoni, L.; Race, M. Soil Washing Optimization, Recycling of the Solution, and Ecotoxicity Assessment for the Remediation of Pb-Contaminated Sites Using EDDS. Sustainability 2018, 10, 636. [Google Scholar] [CrossRef] [Green Version]
- Quina, M.J.; Bontempi, E.; Bogush, A.; Schlumberger, S.; Weibel, G.; Braga, R.; Funari, V.; Hyks, J.; Rasmussen, E.; Lederer, J. Technologies for the management of MSW incineration ashes from gas cleaning: New perspectives on recovery of secondary raw materials and circular economy. Sci. Total Environ. 2018, 635, 526–542. [Google Scholar] [CrossRef]
- Zanoletti, A.; Bilo, F.; Depero, L.E.; Zappa, D.; Bontempi, E. The first sustainable material designed for air particulate matter capture: An introduction to Azure Chemistry. J. Environ. Manag. 2018, 218, 355–362. [Google Scholar] [CrossRef]
- Benassi, L.; Dalipi, R.; Consigli, V.; Pasquali, M.; Borgese, L.; Depero, L.E.; Clegg, F.; Bingham, P.A.; Bontempi, E. Integrated management of ash from industrial and domestic combustion: A new sustainable approach for reducing greenhouse gas emissions from energy conversion. Environ. Sci. Pollut. Res. 2017, 24, 14834–14846. [Google Scholar] [CrossRef]
- Guarienti, M.; Gianoncelli, A.; Bontempi, E.; Moscoso Cardozo, S.; Borgese, L.; Zizioli, D.; Mitola, S.; Depero, L.E.; Presta, M. Biosafe inertization of municipal solid waste incinerator residues by COSMOS technology. J. Hazard. Mater. 2014, 279, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Benassi, L.; Pasquali, M.; Zanoletti, A.; Dalipi, R.; Borgese, L.; Depero, L.E.; Vassura, I.; Quina, M.J.; Bontempi, E. Chemical Stabilization of Municipal Solid Waste Incineration Fly Ash without Any Commercial Chemicals: First Pilot-Plant Scaling Up. ACS Sustain. Chem. Eng. 2016, 4, 5561–5569. [Google Scholar] [CrossRef]
- Bontempi, E.; Zacco, A.; Borgese, L.; Gianoncelli, A.; Ardesi, R.; Depero, L.E. A new method for municipal solid waste incinerator (MSWI) fly ash inertization, based on colloidal silica. J. Environ. Monit. 2010, 12, 2093–2099. [Google Scholar] [CrossRef] [PubMed]
- Bosio, A.; Zacco, A.; Borgese, L.; Rodella, N.; Colombi, P.; Benassi, L.; Depero, L.E.; Bontempi, E. A sustainable technology for Pb and Zn stabilization based on the use of only waste materials: A green chemistry approach to avoid chemicals and promote CO2 sequestration. Chem. Eng. J. 2014, 253, 377–384. [Google Scholar] [CrossRef]
- Bosio, A.; Rodella, N.; Gianoncelli, A.; Zacco, A.; Borgese, L.; Depero, L.E.; Bingham, P.A.; Bontempi, E. A new method to inertize incinerator toxic fly ash with silica from rice husk ash. Environ. Chem. Lett. 2013, 11, 329–333. [Google Scholar] [CrossRef]
- Rodella, N.; Pasquali, M.; Zacco, A.; Bilo, F.; Borgese, L.; Bontempi, N.; Tomasoni, G.; Depero, L.E.; Bontempi, E. Beyond waste: New sustainable fillers from fly ashes stabilization, obtained by low cost raw materials. Heliyon 2016, 2, e00163. [Google Scholar] [CrossRef] [Green Version]
- Benassi, L.; Zanoletti, A.; Depero, L.E.; Bontempi, E. Sewage sludge ash recovery as valuable raw material for chemical stabilization of leachable heavy metals. J. Environ. Manag. 2019, 245, 464–470. [Google Scholar] [CrossRef]
- Assi, A.; Federici, S.; Bilo, F.; Zacco, A.; Depero, L.E.; Bontempi, E. Increased sustainability of carbon dioxide mineral sequestration by a technology involving fly ash stabilization. Materials 2019, 12, 2714. [Google Scholar] [CrossRef] [Green Version]
- Zanoletti, A.; Federici, S.; Borgese, L.; Bergese, P.; Ferroni, M.; Depero, L.E.; Bontempi, E. Embodied energy as key parameter for sustainable materials selection: The case of reusing coal fly ash for removing anionic surfactants. J. Clean. Prod. 2017, 141, 230–236. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ponti, J.; Valsesia, A.; La Spina, R.; Zacco, A.; Bontempi, E. Zero-waste approach in municipal solid waste incineration: Reuse of bottom ash to stabilize fly ash. J. Clean. Prod. 2019, 245, 118779. [Google Scholar] [CrossRef]
- Zacco, A.; Gianoncelli, A.; Ardesi, R.; Sacrato, S.; Guerini, L.; Bontempi, E.; Tomasoni, G.; Alberti, M.; Depero, L.E. Use of colloidal silica to obtain a new inert from municipal solid waste incinerator (MSWI) fly ash: First results about reuse. Clean Technol. Environ. Policy 2012, 14, 291–297. [Google Scholar] [CrossRef]
- Stefano, B.; Hrelja, D.; Lorenzetti, A.; Modesti, M.; Mariangela, B.; Alessandra, G.; Depero, L.E.; Elza, B. New resources from waste recovery: Synthesis and properties polymer based composites containing innovative inertized fly ash from municipal solid waste incineration. In Proceedings of the Society of Plastics Engineers—Technical Conference and Exhibition of the Society of Plastics Engineers, ANTEC DUBAI 2014, Dubai, UAE, 21–22 January 2014. [Google Scholar]
- Besco, S.; Bosio, A.; Brisotto, M.; Depero, L.; Lorenzetti, A.; Bontempi, E.; Bonora, R.; Modesti, M. Structural and Mechanical Characterization of Sustainable Composites Based on Recycled and Stabilized Fly Ash. Materials 2014, 7, 5920–5933. [Google Scholar] [CrossRef] [Green Version]
- Besco, S.; Brisotto, M.; Gianoncelli, A.; Depero, L.E.; Bontempi, E.; Lorenzetti, A.; Modesti, M. Processing and properties of polypropylene-based composites containing inertized fly ash from municipal solid waste incineration. J. Appl. Polym. Sci. 2013, 130, 4157–4164. [Google Scholar] [CrossRef]
- Blasenbauer, D.; Huber, F.; Lederer, J.; Quina, M.J.; Blanc-Biscarat, D.; Bogush, A.; Bontempi, E.; Blondeau, J.; Chimenos, J.M.; Dahlbo, H.; et al. Legal situation and current practice of waste incineration bottom ash utilisation in Europe. Waste Manag. 2020, 102, 868–883. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A. Cement Replacement Materials; Properties, Durability, Sustainability; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2014; Volume 7, ISBN 978-3-642-36720-5. [Google Scholar]
- Pandey, R.A.; Biswas, R.; Chakrabarti, T.; Devotta, S. Flue Gas Desulfurization: Physicochemical and Biotechnological Approaches. Crit. Rev. Environ. Sci. Technol. 2005, 35, 571–622. [Google Scholar] [CrossRef]
- Inada, M.; Eguchi, Y.; Enomoto, N.; Hojo, J. Synthesis of zeolite from coal fly ashes with different silica-alumina composition. Fuel 2005, 84, 299–304. [Google Scholar] [CrossRef]
- UNI EN 12457-2:2004. Available online: http://store.uni.com/catalogo/index.php/uni-en-12457-2-2004 (accessed on 10 December 2019).
- Klockenkämper, R.; von Bohlen, A. Total-Reflection X-ray Fluorescence Analysis and Related Methods, 2nd ed.; Klockenkämper, R., von Bohlen, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; ISBN 9781118460276. [Google Scholar]
- Borgese, L.; Dalipi, R.; Riboldi, A.; Bilo, F.; Zacco, A.; Federici, S.; Bettinelli, M.; Bontempi, E.; Depero, L.E. Comprehensive approach to the validation of the standard method for total reflection X-ray fluorescence analysis of water. Talanta 2018, 181, 165–171. [Google Scholar] [CrossRef]
- UNI EN ISO 178:2010 Plastics—Determination of Flexural Properties; ISO: Geneva, Switzerland, 2013.
- Bontempi, E. A new approach for evaluating the sustainability of raw materials substitution based on embodied energy and the CO2 footprint. J. Clean. Prod. 2017, 162, 162–169. [Google Scholar] [CrossRef]
- Michael, A.F. Materials and the Environment; Butterworth-Heinemann: Oxford, UK, 2012; ISBN 9780123859716. [Google Scholar]
- Chou, J.D.; Wey, M.Y.; Liang, H.H.; Chang, S.H. Biotoxicity evaluation of fly ash and bottom ash from different municipal solid waste incinerators. J. Hazard. Mater. 2009, 168, 197–202. [Google Scholar] [CrossRef]
- Seniunaite, J.; Vasarevicius, S. Leaching of Copper, Lead and Zinc from Municipal Solid Waste Incineration Bottom Ash. Energy Procedia 2017, 113, 442–449. [Google Scholar] [CrossRef]
- Zhao, Y. Pollution Control and Resource Recovery: Municipal Solid Wastes Incineration: Bottom Ash and Fly Ash; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128124970. [Google Scholar]
- Rodella, N.; Bosio, A.; Dalipi, R.; Zacco, A.; Borgese, L.; Depero, L.E.; Bontempi, E. Waste silica sources as heavy metal stabilizers for municipal solid waste incineration fly ash. Arab. J. Chem. 2017, 10, S3676–S3681. [Google Scholar] [CrossRef] [Green Version]
- Pasquali, M.; Zanoletti, A.; Benassi, L.; Federici, S.; Depero, L.E.; Bontempi, E. Stabilized biomass ash as a sustainable substitute for commercial P-fertilizers. Land Degrad. Dev. 2018, 29, 2199–2207. [Google Scholar] [CrossRef]
- Struis, R.P.W.J.; Pasquali, M.; Borgese, L.; Gianoncelli, A.; Gelfi, M.; Colombi, P.; Thiaudière, D.; Depero, L.E.; Rizzo, G.; Bontempi, E. Inertisation of heavy metals in municipal solid waste incineration fly ash by means of colloidal silica-a synchrotron X-ray diffraction and absorption study. RSC Adv. 2013, 3, 14339–14351. [Google Scholar] [CrossRef]
- Shi, C.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Ghouleh, Z.; Shao, Y. Turning municipal solid waste incineration into a cleaner cement production. J. Clean. Prod. 2018, 195, 268–279. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Federici, S.; Zacco, A.; Depero, L.E.; Bontempi, E. Bottom ash derived from municipal solid waste and sewage sludge co-incineration: Characterization and reuse. Waste Manag. 2020. Submitted. [Google Scholar]
- Cornacchia, G.; Agnelli, S.; Gelfi, M.; Ramorino, G.; Roberti, R. Reuse of EAF Slag as Reinforcing Filler for Polypropylene Matrix Composites. JOM 2015, 67, 1370–1378. [Google Scholar] [CrossRef]
- Erklig, A.; Alsaadi, M.; Bulut, M. A comparative study on industrial waste fillers affecting mechanical properties of polymer-matrix composites. Mater. Res. Express 2016, 3, 105302. [Google Scholar] [CrossRef]
- Satheesh Raja, R.; Manisekar, K.; Manikandan, V. Study on mechanical properties of fly ash impregnated glass fiber reinforced polymer composites using mixture design analysis. Mater. Des. 2014, 55, 499–508. [Google Scholar] [CrossRef]
- Granta: CES Selector 2019. Available online: https://www.grantadesign.com/it/products/ces/ (accessed on 10 December 2019).
- Bontempi, E. Case study of raw materials substitution: Natural fillers substitution in plastic composites. In SpringerBriefs in Applied Sciences and Technology; Springer: Cham, Switzerland, 2017; pp. 29–61. [Google Scholar]
Figure 1.
Pilot plant built at Municipal Solid Waste Incineration MSWI plant.
Figure 1.
Pilot plant built at Municipal Solid Waste Incineration MSWI plant.
Figure 2.
Specimen preparation: (a) stabilized sample-composite material, (b) CaCO3-composite material.
Figure 2.
Specimen preparation: (a) stabilized sample-composite material, (b) CaCO3-composite material.
Figure 3.
X-ray diffraction (XRD) pattern of stabilized fly ash (FA) after 11 months.
Figure 3.
X-ray diffraction (XRD) pattern of stabilized fly ash (FA) after 11 months.
Figure 4.
Stress-strain curves obtained from flexural tests for different composites.
Figure 4.
Stress-strain curves obtained from flexural tests for different composites.
Figure 5.
Flexural modulus as a function of filler content.
Figure 5.
Flexural modulus as a function of filler content.
Figure 6.
Flexural strength as a function of filler content.
Figure 6.
Flexural strength as a function of filler content.
Figure 7.
Scanning electron microscope (SEM) images of stabilized sample-composite material 50% wt (a) and CaCO3 composite material 50% wt (b) at magnification 2000×.
Figure 7.
Scanning electron microscope (SEM) images of stabilized sample-composite material 50% wt (a) and CaCO3 composite material 50% wt (b) at magnification 2000×.
Figure 8.
Energy dispersive X-ray spectroscopy (EDX) maps of Ca distribution of stabilized sample-composite: 10% (a), 50% (b), 70% (c) and CaCO3-composite 10% (d), 50% (e), 70% (f).
Figure 8.
Energy dispersive X-ray spectroscopy (EDX) maps of Ca distribution of stabilized sample-composite: 10% (a), 50% (b), 70% (c) and CaCO3-composite 10% (d), 50% (e), 70% (f).
Figure 9.
Embodied energy, primary productions (MJ/kg) vs. price (EUR/kg) of various materials and composites.
Figure 9.
Embodied energy, primary productions (MJ/kg) vs. price (EUR/kg) of various materials and composites.
Table 1.
Results of the total reflection X-ray Fluorescence (TXRF) analysis and pH values of leaching test of raw powders. Values are expressed as the average ± standard deviation of three TXRF measurements. Relative sensitivities for elements with * were calculated based on a calibration curve (see the experimental section). LOD—lower detection limit.
Table 1.
Results of the total reflection X-ray Fluorescence (TXRF) analysis and pH values of leaching test of raw powders. Values are expressed as the average ± standard deviation of three TXRF measurements. Relative sensitivities for elements with * were calculated based on a calibration curve (see the experimental section). LOD—lower detection limit.
| | Elemental Concentration (mg/L) |
|---|
| Elements | FA | BA | CFA | FGD |
|---|
| pH | 12.18 | 12.2 | 12.01 | 12.68 |
|---|
| P * | 24 | ± | 17 | 9 | ± | 3 | 6 | ± | 2 | 9 | ± | 2 |
| S * | 311 | ± | 73 | 131 | ± | 21 | 170 | ± | 10 | 320 | ± | 50 |
| Cl | 5029 | ± | 3222 | 318 | ± | 185 | 1.74 | ± | 1.06 | 400 | ± | 50 |
| K | 702 | ± | 412 | 81 | ± | 21 | 31 | ± | 2 | 10 | ± | 2 |
| Ca | 3488 | ± | 1548 | 979 | ± | 174 | 450 | ± | 30 | 1700 | ± | 200 |
| Cr | <LOD | <LOD | 0.21 | ± | 0.03 | <LOD |
| Mn | <LOD | <LOD | <LOD | <LOD |
| Fe | 0.62 | ± | 0.40 | 0.07 | ± | 0.03 | 0.27 | ± | 0.01 | 0.12 | ± | 0.04 |
| Cu | 0.38 | ± | 0.08 | 5.6 | ± | 0.7 | <LOD | 0.83 | ± | 0.28 |
| Zn | 8.8 | ± | 4.3 | 0.66 | ± | 0.16 | 0.21 | ± | 0.14 | 0.10 | ± | 0.04 |
| Br | 193 | ± | 13 | 1.88 | ± | 0.20 | 0.20 | ± | 0.03 | 1.67 | ± | 0.18 |
| Rb | 7.6 | ± | 0.9 | 0.19 | ± | 0.02 | <LOD | <LOD |
| Sr | 16 | ± | 1 | 5.5 | ± | 0.6 | 3.43 | ± | 0.18 | 2.84 | ± | 0.34 |
| Ba | 1.28 | ± | 0.96 | 0.78 | ± | 0.49 | 0.76 | ± | 0.40 | <LOD |
| Pb | 35 | ± | 2 | 1.60 | ± | 0.33 | <LOD | <LOD |
Table 2.
Results of the TXRF analysis and pH values of leachate stabilized sample after 1, 2, 3 and 11 months. Values are expressed as the average ± standard deviation of three TXRF measurements. Relative sensitivities for elements with * were calculated based on a calibration curve (see the experimental section). LOD—lower detection limit.
Table 2.
Results of the TXRF analysis and pH values of leachate stabilized sample after 1, 2, 3 and 11 months. Values are expressed as the average ± standard deviation of three TXRF measurements. Relative sensitivities for elements with * were calculated based on a calibration curve (see the experimental section). LOD—lower detection limit.
| Stabilized Samples |
|---|
| Time | 1 M | 2 M | 3 M | 11 M |
|---|
| pH | 12.1 | 10.2 | 9.87 | 9.7 |
|---|
| P * | 62 | ± | 8 | 24 | ± | 2 | 35 | ± | 4 | 33 | ± | 14 |
| S * | 297 | ± | 23 | 512 | ± | 58 | 597 | ± | 56 | 491 | ± | 104 |
| Cl | 3793 | ± | 714 | 2117 | ± | 128 | 2865 | ± | 341 | 1825 | ± | 185 |
| K | 522 | ± | 195 | 284 | ± | 34 | 303 | ± | 21 | 195 | ± | 39 |
| Ca | 3117 | ± | 643 | 1834 | ± | 102 | 2203 | ± | 47 | 1432 | ± | 278 |
| Fe | 0.13 | ± | 0.08 | 0.11 | ± | 0.002 | 0.51 | ± | 0.14 | 0.21 | ± | 0.06 |
| Cu | 0.04 | ± | 0.01 | 0.05 | ± | 0.03 | 0.16 | ± | 0.03 | 0.29 | ± | 0.11 |
| Zn | 0.47 | ± | 0.22 | 0.06 | ± | 0.01 | 0.1 | ± | 0.06 | 0.11 | ± | 0.05 |
| Br | 71 | ± | 29 | 88 | ± | 13 | 98 | ± | 15 | 87 | ± | 4 |
| Rb | 2.5 | ± | 1.2 | 3.5 | ± | 0.9 | 4.2 | ± | 0.5 | 3.6 | ± | 1.1 |
| Sr | 12 | ± | 3 | 11 | ± | 1 | 12 | ± | 1 | 13 | ± | 2 |
| Ba | 1.22 | ± | 0.85 | 0.36 | ± | 0.05 | 0.72 | ± | 0.35 | 0.6 | ± | 0.36 |
| Pb | 2.6 | ± | 0.1 | <LOD | <LOD | <LOD |
Table 3.
Chemical characterization by SEM analysis of epoxy resin (ER), CaCO3-composite (CaCO3) and stabilized sample-composite (SSC).
Table 3.
Chemical characterization by SEM analysis of epoxy resin (ER), CaCO3-composite (CaCO3) and stabilized sample-composite (SSC).
| Samples | Elemental Concentration (%) |
|---|
| C | O | Si | Cl | Ca | Al | Mg | K | S |
|---|
| 100% ER | 66.34 | 29.75 | 2.18 | 1.16 | 0.57 | − | − | − | − |
| 10% CaCO3 | 66.46 | 29.22 | 1.92 | 0.65 | 1.74 | − | − | − | − |
| 30% CaCO3 | 57.91 | 30.3 | 8.44 | − | 3.63 | − | − | − | − |
| 50% CaCO3 | 58.37 | 34.21 | 1.35 | 0.52 | 5.55 | − | − | − | − |
| 70% CaCO3 | 32.02 | 38.42 | 2.82 | 0.26 | 26.48 | − | − | − | − |
| 9% SSC | 66.81 | 25.96 | 5.04 | 1.07 | 1.12 | − | − | − | − |
| 10% SSC | 68.53 | 29.04 | 0.60 | 1.04 | 0.80 | − | − | − | − |
| 30% SSC | 63.93 | 31.88 | 1.16 | 1.37 | 1.67 | − | − | − | − |
| 50% SSC | 57.77 | 26.84 | 2.86 | 4.32 | 7.53 | 0.68 | − | − | − |
| 70% SSC | 47.68 | 30.47 | 2.08 | 5.68 | 10.43 | 1.02 | 0.38 | 0.51 | 1.77 |
Table 4.
SUB-RAW Indices were calculated comparing the obtained composite using stabilized FA sample with other materials.
Table 4.
SUB-RAW Indices were calculated comparing the obtained composite using stabilized FA sample with other materials.
| Materials | SUB-RAW Index–New Filler |
|---|
| Sandstone | 1.24 |
| Calcite | 0.83 |
| Talc | 2.24 |
| Resin | 3.31 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).