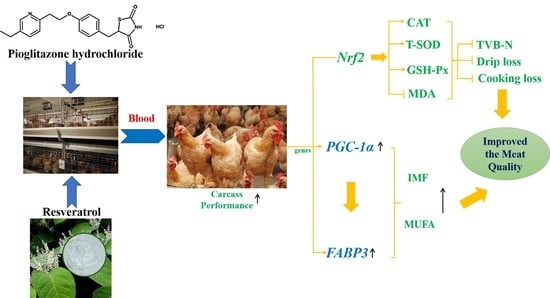
Dietary Supplementation with Pioglitazone Hydrochloride and Resveratrol Improves Meat Quality and Antioxidant Capacity of Broiler Chickens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design, Diets and Growth Performance
2.3. Serum Biochemical Indices
2.4. Carcass Performance and Meat Quality
2.5. Determination of the Fatty Acid Composition
2.6. Determination of Muscle Antioxidant Abilities and ROS
2.7. Determination of mRNA Abundances
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Serum Biochemical Indices
3.3. Carcass Performance and Meat Quality
3.4. Determination of the Fatty Acid Composition
3.5. Determination of Muscle Antioxidant Abilities and ROS
3.6. Determination of mRNA Abundances
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kouba, M.; Mourot, J. A review of nutritional effects on fat composition of animal products with special emphasis on n-3 polyunsaturated fatty acids. Biochimie 2011, 93, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Bonoli, M.; Caboni, M.F.; Rodriguez-Estrada, M.T.; Lercker, G. Effect of feeding fat sources on the quality and composition of lipids of precooked ready-to-eat fried chicken patties. Food Chem. 2007, 101, 1327–1337. [Google Scholar] [CrossRef]
- Kim, J.E.; Clark, R.M.; Park, Y.; Lee, J.; Fernandez, M.L. Lutein decreases oxidative stress and inflammation in liver and eyes of guinea pigs fed a hypercholesterolemic diet. Nutr. Res. Pract. 2012, 6, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Engel, E.J.; Ratel, J.; Bouhlel, C.; Planche, C.; Meurillon, M. Novel approaches to improving the chemical safety of the meat chain towards toxicants. Meat Sci. 2015, 109, 75–85. [Google Scholar] [CrossRef]
- Fung, D.Y.; Toldra, F. Microbial hazards in food: Food-borne infections and intoxications. In Handbook of Meat Processing; Blackwell Publishing: Hoboken, NJ, USA, 2010; pp. 481–500. [Google Scholar] [CrossRef]
- Dave, D.; Ghaly, A.E. Meat spoilage mechanisms and preservation techniques: A critical review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar] [CrossRef] [Green Version]
- Fürrnsinn, C.; Waldhausl, W. Thiazolidinediones: Metabolic actions in vitro. Diabetologia 2002, 45, 1211–1223. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care 2014, 37 (Suppl. S1), S14–S80. [Google Scholar] [CrossRef] [Green Version]
- Mirmiranpour, H.; Mousavizadeh, M.; Noshad, S.; Ghavami, M.; Ebadi, M.; Ghasemiesfe, M. Comparative effects of pioglitazone and metformin on oxidative stress markers in newly diagnosed type 2 diabetes patients: A randomized clinical trial. J. Diabetes Complicat. 2013, 27, 501–507. [Google Scholar] [CrossRef]
- Rodriguez, A.; Reviriego, J.; Karamanos, V.; del Canizo, F.J.; Vlachogiannis, N.; Drossinos, V. Management of cardiovascular risk factors with pioglitazone combination therapies in type 2 diabetes: An observational cohort study. Cardiovasc. Diabetol. 2011, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, A.; Watanabe, M.; Fujita, Y.; Saigenji, K.; Kuwao, S.; Takahashi, H. An autopsy case of troglitazone-induced fulminant hepatitis. Diabetes Care 1998, 21, 2140–2143. [Google Scholar] [CrossRef]
- Chabowski, A.; Endzian-Piotrowska, M.; Nawrocki, A.; Górski, J. Not only accumulation, but also saturation status of intramuscular lipids is significantly affected by pparγ activation. Acta Physiol. 2012, 205, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Muurling, M.; Mensink, R.P.; Pijl, H.; Romijn, J.A.; Havekes, L.M.; Voshol, P.J. Rosiglitazone improves muscle insulin sensitivity, irrespective of increased triglyceride content, in ob/ob mice. Metabolism 2003, 52, 1078–1083. [Google Scholar] [CrossRef]
- Lessard, S.J.; Lo Giudice, S.L.; Lau, W.; Reid, J.J.; Turner, N.; Febbraio, M.A.; Hawley, J.A.; Watt, M.J. Rosiglitazone enhances glucose tolerance by mechanisms other than reduction of fatty acid accumulation within skeletal muscle. Endocrinology 2004, 145, 5665–5670. [Google Scholar] [CrossRef] [PubMed]
- Poulos, S.; Hausman, G. A comparison of thiazolidinedione-induced adipogenesis and myogenesis in stromal-vascular cells from subcutaneous adipose tissue or semitendinosus muscle of postnatal pigs. J. Anim. Sci. 2006, 84, 1076–1082. [Google Scholar] [CrossRef]
- George, R.B.; Natasha, B.K.; Gary, F.B.; Corinne, E.C.; Yiming, L.; Laura, M.G.; Joselita, S.; Limin, P.; Francisco, P.; Denise, U. The effects of thiazolidinediones on human bone marrow stromal cell differentiation in vitro and in thiazolidinedione-treated patients with type 2 diabetes. Transl. Res. 2012, 161, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Machado, M.M.; Bassani, T.B.; Cóppola-Segovia, V.; Moura, E.L.; Zanata, S.M.; Andreatini, R.; Vital, M.A. PPAR-γ agonist pioglitazone reduces microglial proliferation and nf-κb activation in the substantia nigra in the 6-hydroxydopamine model of parkinson’s disease. Pharmacol. Rep. 2019, 71, 556–564. [Google Scholar] [CrossRef]
- MacKellar, J.; Cushman, S.W.; Periwal, V. Differential effects of thiazolidinediones on adipocyte growth and recruitment in zucker fatty rats. PLoS ONE 2009, 4, e8196. [Google Scholar] [CrossRef] [Green Version]
- Arevalo-Turrubiarte, M.; Gonzalez-Davalos, L.; Yabuta, A.; Garza, J.D.; Davalos, J.L.; Mora, O.; Shimada, A. Effect of 2, 4-thiazolidinedione on limousin cattle growth and on muscle and adipose tissue metabolism. PPAR Res. 2012. [Google Scholar] [CrossRef]
- Schoenberg, K.M.; Overton, T.R. Effects of plane of nutrition and 2, 4-thiazolidinedione on insulin responses and adipose tissue gene expression in dairy cattle during late gestation. J. Dairy Sci. 2011, 94, 6021–6035. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.L.; Gao, C.Q.; Wang, Q.; Zhang, Z.M.; Xu, Y.L.; Li, H.C.; Yan, H.C.; Wang, X.Q. Effects of pioglitazone hydrochloride and vitamin E on meat quality, antioxidant status and fatty acid profiles in finishing pigs. Meat Sci. 2018, 145, 340–346. [Google Scholar] [CrossRef]
- Chen, X.; Feng, Y.; Yang, W.J.; Shu, G.; Jiang, Q.Y.; Wang, X.Q. Effects of dietary thiazolidinedione supplementation on growth performance, intramuscular fat and related genes mRNA abundance in the longissimus dorsi muscle of finishing pigs. Asian Australas. J. Anim. Sci. 2013, 26, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Ognik, K.; Cholewińska, E.; Sembratowicz, I.; Grela, E.; Czech, A. The potential of using plant antioxidants to stimulate antioxidant mechanisms in poultry. Worlds Poult. Sci. J. 2016, 72, 291–298. [Google Scholar] [CrossRef]
- Sahin, K.; Akdemir, F.; Orhan, C.; Tuzcu, M.; Hayirli, A.; Sahin, N. Effects of dietary resveratrol supplementation on egg production and antioxidant status. Poult. Sci. 2010, 89, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; He, J.H.; Xie, H.B.; Yang, Y.S.; Li, J.C.; Zou, Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2014, 93, 54–62. [Google Scholar] [CrossRef]
- Rubiolo, J.A.; Vega, F.V. Resveratrol protects primary rat hepatocytes against necrosis induced by reactive oxygen species. Biomed. Pharmacother. 2008, 62, 606–612. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, W.; Tan, H.Z.; Xu, G.F.; Zhang, X.B.; Wei, S.; Wang, X.Q. Effects of outdoor access on growth performance, carcass composition, and meat characteristics of broiler chickens. Poult. Sci. 2013, 92, 435–443. [Google Scholar] [CrossRef]
- Goulas, A.E.; Kontominas, M.G. Effect of salting and smoking-method on the keeping quality of chub mackerel (Scomber japonicus): Biochemical and sensory attributes. Food Chem. 2005, 93, 511–520. [Google Scholar] [CrossRef]
- Sullivan, K.; El-Hoss, J.; Quinlan, K.G.; Deo, N.; Garton, F.; Seto, J.T. NF1 is a critical regulator of muscle development and metabolism. Hum. Mol. Genet. 2014, 23, 1250–1259. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.L.; Wang, Q.; Zhang, Z.M. Dietary Supplementation with Pioglitazone Hydrochloride and Chromium Methionine Improves Growth Performance, Meat Quality, and Antioxidant Ability in Finishing Pigs. J. Agric. Food Chem. 2018, 66, 4345–4351. [Google Scholar] [CrossRef]
- Slover, H.T.; Lanza, E. Quantitative analysis of food fatty acids by capillary gas chromatography. J. Am. Oil Chem. Soc. 1979, 56, 933–943. [Google Scholar] [CrossRef]
- Wan, X.; Ahmad, H.; Zhang, L.; Wang, Z.; Wang, T. Dietary enzymatically treated Artemisia annua L. improves meat quality, antioxidant capacity and energy status of breast muscle in heat-stressed broilers. J. Sci. Food Agric. 2018, 98, 3715–3721. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.Y.; Jiang, S.Q.; Jiang, Z.Y.; Zheng, C.T.; Li, L.; Ruan, D.; Lin, X.J. Effects of high peanut meal with different crude protein level supplemented with amino acids on performance, carcass traits and nitrogen retention of Chinese Yellow broilers. J. Anim. Physiol. Anim. Nutr. 2016, 100, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Jiang, W.; Tan, H.Z.; Zhang, D.X.; Zhang, H.J.; Wei, S.; Yan, H.C. Effects of breed and dietary nutrient density on the growth performance, blood metabolite, and genes expression of target of rapamycin (TOR) signaling pathway of female broiler chickens. J. Anim. Physiol. Anim. Nutr. 2013, 97, 797–806. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.J.; Eckhardt, M.; Gagen, K.; Dong, M.; Chen, W.; Laurent, D. Effects of pioglitazone on adipose tissue remodeling within the setting of obesity and insulin resistance. Diabetes 2001, 50, 1863–1871. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Luo, J.; Yu, B.; Zheng, P.; Huang, Z.; Mao, X.; He, J.; Yu, J.; Chen, J.; Chen, D. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 2015, 102, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, J.; Li, L.; Zheng, J.; Shi, Y.; Le, G. Regulatory effects of resveratrol on glucose metabolism and T-lymphocyte subsets in the development of high-fat diet-induced obesity in C57BL/6 mice. Food Funct. 2014, 5, 1452–1463. [Google Scholar] [CrossRef]
- Khan, M.A.; Peter, J.V.; Xue, J.L. A prospective, randomized comparison of the metabolic effects of pioglitazone or rosiglitazone in patients with type 2 diabetes who were previously treated with troglitazone. Diabetes Care 2002, 25, 708–711. [Google Scholar] [CrossRef] [Green Version]
- Boyle, P.J.; King, A.B.; Olansky, L. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: A retrospective review of randomly selected medical records. Clin. Ther. 2002, 24, 378–396. [Google Scholar] [CrossRef]
- Mazur-Kuśnirek, M.; Antoszkiewicz, Z.; Lipiński, K.; Fijałkowska, M.; Purwin, C.; Kotlarczyk, S. The effect of polyphenols and vitamin E on the antioxidant status and meat quality of broiler chickens fed diets naturally contaminated with ochratoxin A. Arch. Anim. Nutr. 2019, 73, 431–444. [Google Scholar] [CrossRef]
- Cai, J.R.; Chen, Q.S.; Wan, X.M.; Zhao, J.W. Determination of total volatile basic nitrogen (TVB-N) content and Warner–Bratzler shear force (WBSF) in pork using Fourier transform near infrared (FT-NIR) spectroscopy. Food Chem. 2011, 126, 1354–1360. [Google Scholar] [CrossRef]
- Rukchon, C.; Nopwinyuwong, A.; Trevanich, S.; Jinkarn, T.; Suppakul, P. Development of a food spoilage indicator for monitoring freshness of skinless chicken breast. Talanta 2014, 130, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lin, Y.; Jiang, Z.; Zheng, C.; Zhou, G.; Yu, D.; Cao, T.; Wang, J.; Chen, F. Dietary arginine supplementation enhances antioxidative capacity and improves meat quality of finishing pigs. Amino Acids 2010, 38, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Veleba, J.; Kopecky, J.; Janovska, P.; Kuda, O.; Horakova, O.; Malinska, H.; Hajek, M. Combined intervention with pioglitazone and n-3 fatty acids in metformin-treated type 2 diabetic patients: Improvement of lipid metabolism. Nutr. Metab. 2015, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Li, F.N.; Yang, H.S.; Duan, Y.H.; Yin, Y.L. Myostatin regulates preadipocyte differentiation and lipid metabolism of adipocyte via ERK1/2. Cell Biol. Int. 2011, 35, 1141–1146. [Google Scholar] [CrossRef]
- Hondares, E.; Mora, O.; Yubero, P.; de la Concepción, M.R.; Iglesias, R.; Giralt, M.; Villarroya, F. Thiazolidinediones and rexinoids induce peroxisome proliferator-activated receptor-coactivator (PGC)-1α gene transcription: An autoregulatory loop controls PGC-1α expression in adipocytes via peroxisome proliferator-activated receptor-α coactivation. Endocrinology 2006, 147, 2829–2838. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Luo, J.; Yu, B.; Chen, J.; Chen, D. Effects of resveratrol on lipid metabolism in muscle and adipose tissues: A reevaluation in a pig model. J. Funct. Foods 2015, 14, 590–595. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Arulselvan, P.; Subramanian, S.P. Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultrastructural changes of pancreatic b-cells in experimental diabetes in rats. Chem. Biol. Interact. 2007, 165, 155–164. [Google Scholar] [CrossRef]
- Mujahid, A.; Akiba, Y.; Warden, C.H.; Toyomizu, M. Sequential changes in superoxide production, anion carriers and substrate oxidation in skeletal muscle mitochondria of heatstressed chickens. FEBS Lett. 2007, 581, 3461–3467. [Google Scholar] [CrossRef] [Green Version]
- Paulo, L.; Ferreira, S.; Gallardo, E.; Queiroz, J.A.; Domingues, F. Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World J. Microbiol. Biotechnol. 2010, 26, 1533–1538. [Google Scholar] [CrossRef]
- Alagawany, M.M.; Farag, M.R.; Dhama, K.; Abd El-Hack, M.E.; Tiwari, R.; Alam, G.M. Mechanisms and beneficial applications of resveratrol as feed additive in animal and poultry nutrition: A review. Int. J. Pharmacol. 2015, 11, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chan, F.; Sun, H.; Yan, J.; Fan, D.; Zhao, D.; An, J.; Zhou, D. Resveratrol protects human keratinocytes HaCaT cells from uVA-induced oxidative stress damage by downregulating Keap1 expression. Eur. J. Pharmacol. 2011, 650, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Sgambato, A.; Ardito, R.; Faraglia, B.; Boninsegna, A.; Wolf, F.I.; Cittadini, A. Resveratrol, a natural phenolic compound, inhibits cell proliferation and prevents oxidative DNA damage. Mutat. Res. 2001, 496, 171–180. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasubramanian, P.; Longo, V.D. Linking Klotho, NRF2, MAP kinases and aging. Aging 2010, 2, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Item | Treatment | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| Control | PGZ | RES | PGZ + RES | |||
| IBW (g) | 1623.6 | 1624.2 | 1623.7 | 1624.1 | 8.24 | 0.303 |
| FBW (g) | 2112.3 b | 2163.1 a | 2119.6 b | 2168.5 a | 10.32 | <0.05 |
| ADFI (g) | 93.4 | 94.2 | 94.8 | 94.9 | 2.01 | 0.647 |
| ADG (g) | 17.4 b | 19.2 a | 17.7 b | 19.4 a | 0.51 | <0.05 |
| F/G (g/g) | 5.37 a | 4.91 b | 5.34 a | 4.89 b | 0.09 | <0.05 |
| Item | Treatment | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| Control | PGZ | RES | PGZ + RES | |||
| SUN (mmol/L) | 0.61 | 0.50 | 0.51 | 0.59 | 0.06 | 0.331 |
| LDL-C (mmol/L) | 0.91 a | 0.71 b | 0.64 b | 0.36 c | 0.04 | <0.05 |
| CHO (mmol/L) | 3.30 a | 2.97 b | 3.20 a | 2.86 b | 0.11 | <0.05 |
| TP (g/L) | 45.53 | 46.96 | 46.70 | 48.07 | 1.45 | 0.251 |
| TG (mmol/L) | 1.73 | 1.62 | 1.78 | 1.89 | 0.57 | 0.734 |
| HDL-C (mmol/L) | 1.73 b | 2.31 a | 1.67 b | 2.56 a | 0.08 | <0.05 |
| Item | Treatment | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| Control | PGZ | RES | PGZ + RES | |||
| Dressing percentage (%) | 90.96 b | 91.71 a | 91.54 a | 91.73 a | 0.20 | <0.05 |
| Semi-eviscerated yield (%) | 86.53 b | 87.28 a | 87.24 a | 87.25 a | 0.19 | <0.05 |
| Eviscerated yield (%) | 69.75 | 69.80 | 70.33 | 70.81 | 0.33 | 0.817 |
| Breast muscle yield (%) | 14.98 | 14.67 | 14.84 | 14.12 | 0.78 | 0.520 |
| Thigh muscle yield (%) | 17.46 | 16.71 | 16.88 | 16.2 | 0.56 | 0.793 |
| Abdominal fat (%) | 11.15 a | 10.13 b | 10.12 b | 9.40 b | 0.14 | <0.05 |
| Item | Treatment | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Control | PGZ | RES | PGZ + RES | ||||
| Shear force (N) | 23.42 | 23.44 | 23.58 | 23.23 | 0.47 | 0.935 | |
| Drip loss (%) | 1.43 a | 1.41 a | 1.23 b | 1.20 b | 0.05 | <0.05 | |
| Cooking loss (%) | 22.25 a | 22.85 a | 19.83 b | 19.35 b | 0.76 | <0.05 | |
| pH45 min | 6.02 | 5.94 | 6.06 | 5.98 | 0.06 | 0.305 | |
| pH24 h | 5.82 b | 5.80 b | 5.91 a | 5.94 a | 0.03 | <0.05 | |
| Color at 45 min | L* | 52.44 | 51.93 | 51.10 | 52.21 | 0.51 | 0.532 |
| A* | 12.69 | 12.82 | 12.32 | 12.71 | 0.44 | 0.891 | |
| B* | 7.39 | 7.49 | 8.17 | 8.10 | 0.67 | 0.589 | |
| Color at 24 h | L* | 51.01 | 50.33 | 50.11 | 50.36 | 0.78 | 0.354 |
| A* | 11.04 b | 10.87 b | 11.76 a | 11.84 a | 0.16 | <0.05 | |
| B* | 8.23 | 8.45 | 8.56 | 10.29 | 0.43 | 0.197 | |
| Intramuscular fat (%) | 3.18 b | 3.88 a | 3.22 b | 4.21 a | 0.15 | <0.01 | |
| Item | Treatment | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| Control | PGZ | RES | PGZ + RES | |||
| C14:0 | 0.60 | 0.64 | 0.59 | 0.59 | 0.02 | 0.174 |
| C16:0 | 23.16 | 23.01 | 22.76 | 23.03 | 0.42 | 0.527 |
| C16:1 | 2.91 | 2.83 | 2.91 | 3.01 | 0.17 | 0.314 |
| C18:0 | 8.58 | 8.39 | 8.18 | 8.16 | 0.33 | 0.937 |
| C18:1ω9 | 37.25 b | 38.90 a | 39.03 a | 39.09 a | 0.37 | <0.05 |
| C18:2ω6 | 20.99 | 20.87 | 20.39 | 21.08 | 0.25 | 0.149 |
| C18:3ω6 | 0.15 b | 0.18 a | 0.15 b | 0.19 a | 0.01 | <0.05 |
| C18:3ω3 | 1.50 | 1.51 | 1.49 | 1.48 | 0.03 | 0.537 |
| C22:0 | 0.24 | 0.21 | 0.22 | 0.21 | 0.01 | 0.217 |
| C20:3ω3 | 0.30 b | 0.39 a | 0.32 b | 0.41 a | 0.15 | <0.05 |
| C20:4ω6 | 1.73 | 1.69 | 1.74 | 1.67 | 0.15 | 0.111 |
| SFA | 32.59 | 32.25 | 31.75 | 31.99 | 0.28 | 0.218 |
| MUFA | 40.16 b | 41.73 a | 41.94 a | 42.10 a | 0.33 | <0.01 |
| PUFA | 24.67 | 24.64 | 24.09 | 24.83 | 0.35 | 0.379 |
| Item | Treatment | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| Control | PGZ | RES | PGZ + RES | |||
| ROS (% of Control) | 1.00 a | 0.83 a | 0.41 b | 0.35 b | 0.12 | <0.05 |
| T-AOC (U/mg prot) | 0.20 | 0.21 | 0.21 | 0.25 | 0.07 | 0.978 |
| CAT (U/mg prot) | 2.01 b | 2.05 b | 2.63 a | 2.65 a | 0.15 | <0.05 |
| GSH-Px (U/mg prot) | 8.43 b | 8.27 b | 12.33 a | 12.78 a | 0.43 | <0.05 |
| T-SOD (U/mg prot) | 34.01 b | 33.10 b | 39.26 a | 40.23 a | 0.37 | <0.05 |
| MDA (nmol/mg prot) | 5.03 a | 4.71 a | 3.85 b | 3.72 b | 0.27 | <0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Jin, C.; Jiang, S.; Wang, X.; Yan, H.; Tan, H.; Gao, C. Dietary Supplementation with Pioglitazone Hydrochloride and Resveratrol Improves Meat Quality and Antioxidant Capacity of Broiler Chickens. Appl. Sci. 2020, 10, 2452. https://doi.org/10.3390/app10072452
Zhang F, Jin C, Jiang S, Wang X, Yan H, Tan H, Gao C. Dietary Supplementation with Pioglitazone Hydrochloride and Resveratrol Improves Meat Quality and Antioxidant Capacity of Broiler Chickens. Applied Sciences. 2020; 10(7):2452. https://doi.org/10.3390/app10072452
Chicago/Turabian StyleZhang, Fan, Chenglong Jin, Shiguang Jiang, Xiuqi Wang, Huichao Yan, Huize Tan, and Chunqi Gao. 2020. "Dietary Supplementation with Pioglitazone Hydrochloride and Resveratrol Improves Meat Quality and Antioxidant Capacity of Broiler Chickens" Applied Sciences 10, no. 7: 2452. https://doi.org/10.3390/app10072452
APA StyleZhang, F., Jin, C., Jiang, S., Wang, X., Yan, H., Tan, H., & Gao, C. (2020). Dietary Supplementation with Pioglitazone Hydrochloride and Resveratrol Improves Meat Quality and Antioxidant Capacity of Broiler Chickens. Applied Sciences, 10(7), 2452. https://doi.org/10.3390/app10072452