Legumes as Functional Food for Cardiovascular Disease
Abstract
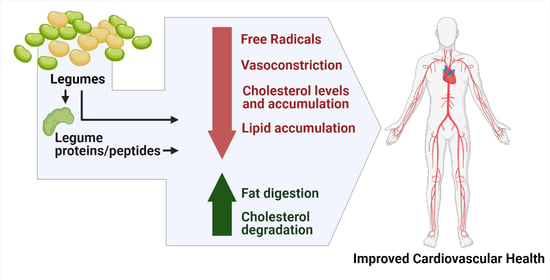
:1. Introduction
2. General Characteristics of Legumes
2.1. Legumes Taxonomy and Distribution
2.2. Biochemical Composition of Legumes
3. Legumes as Functional Food for Cardiovascular Diseases
3.1. Soybean (Glycine Max L.)
3.1.1. Benefits of Soybeans Relating to Cardiovascular Health
Cardiovascular Benefits of Soybean Proteins
Cardiovascular Benefits of Soybean 7S and 11S Globulin Proteins
Cardiovascular Benefits of Soybean 7S β-Conglycinin
Cardiovascular Benefits of Soybean 11S Globulin Peptides
3.1.2. Soybean Peptides and Their Associated Health Benefits
3.1.3. Soybean Lunasin
3.2. Lupin (Lupinus spp.)
3.3. Pea (Pisum Sativum L.)
3.4. Cowpea (Vigna Unguiculata (L.) Walp.)
3.5. Jack Bean (Canavalia Ensiformis (L.) DC.)
3.6. Mungbean (Vigna Radiata L.)
3.7. Chickpea (Cicer Aretinum L.)
3.8. Lentil (Lens Culinaris Medik.)
3.9. Other Legumes
4. Peptide Structure, Length, and Hydrophobicity Analysis of Legume Peptides with Associated Cardiovascular Benefits
5. Gene Network Analyses
6. The Mechanism of Action (MOA) of Legumes Proteins and Peptides Associated with Cardiovascular Health
7. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jakubczyk, A.; Karaś, M.; Rybczyńska-Tkaczyk, K.; Zielińska, E.; Zieliński, D. Current Trends of Bioactive Peptides—New Sources and Therapeutic Effect. Foods 2020, 9, 846. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Potential Role of Bioactive Peptides in Prevention and Treatment of Chronic Diseases: A Narrative Review. Br. J. Pharmacol. 2017, 174, 1378–1394. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases (CVD). 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 6 September 2020).
- Kypreos, K.E.; Bitzur, R.; Karavia, E.A.; Xepapadaki, E.; Panayiotakopoulos, G.; Constantinou, C. Pharmacological Management of Dyslipidemia in Atherosclerosis: Limitations, Challenges, and New Therapeutic Opportunities. Angiology 2018, 70, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Patoulias, D.; Stavropoulos, K.; Imprialos, K.; Athyros, V.; Doumas, M.; Karagiannis, A. Pharmacological Management of Cardiac Disease in Patients with Type 2 Diabetes: Insights into Clinical Practice. Curr. Vasc. Pharmacol. 2020, 18, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.; Ajmal, I.; Naz, T.; Fazili, A.; Bai, X.; Song, Y. Bioactive Functional Foods for Cardiovascular Diseases. Am. J. Biochem. Biotechnol. 2020, 16, 354–369. [Google Scholar] [CrossRef]
- Erdmann, K.; Cheung, B.W.Y.; Schröder, H. The Possible Roles of Food-Derived Bioactive Peptides in Reducing the Risk of Cardiovascular Disease. J. Nutr. Biochem. 2008, 19, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Elmaliklis, I.-N.; Liveri, A.; Ntelis, B.; Paraskeva, K.; Goulis, I.; Koutelidakis, A. Increased Functional Foods’ Consumption and Mediterranean Diet Adherence May Have a Protective Effect in the Appearance of Gastrointestinal Diseases: A Case–Control Study. Medicines 2019, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Functional Food: What Are They? And Why Are They So Popular? Acta Sci. Nutr. Health 2018, 2, 26–27. [Google Scholar]
- Maphosa, Y.; Jideani, V. The Role of Legumes in Human Nutrition. In Functional Food—Improve Health through Adequate Food Maria Chavarri Hueda; Department of Food Science and Technology, Cape Peninsula University of Technology: Bellville, South Africa, 2017; pp. 103–122. ISBN 978-953-51-3440-4. [Google Scholar]
- Trinidad, T.P.; Mallillin, A.C.; Loyola, A.S.; Sagum, R.S.; Encabo, R.R. The Potential Health Benefits of Legumes as a Good Source of Dietary Fibre. Br. J. Nutr. 2010, 103, 569–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo, I.; Angelia, M.; Torio, M.; Belina-Aldemita, M. Antihypertensive Property of the Peptic and Chymotryptic Hydrolysates Derived from the Crude Protein Extract of Okra [Abelmoschus esculentus (L.) Moench} Seeds. Int. Food. Res. J. 2017, 24, 2586–2592. [Google Scholar]
- De Leon, R.; Torio, M.; Manalo, M.; Aguda, R. Isolation, Purification and Characterization of the Major Storage Protein in Cowpea (Vigna unguiculata) Seed with Bioactive Peptides Exhibiting Antiangiotensin I-Converting Enzyme Activity. In Proceedings of the 42nd Annual Convention of the Kapisanang Kimika ng Pilipinas—Southern Tagalog Chapter, Southeast Asian Regional Center for Graduate Study and Research in Agriculture (SEARCA), University of the Philippines Los Baños, Laguna, Philippines, 29–30 October 2013; p. 26. [Google Scholar]
- Distor, N.; Angelia, M.; San Pascual, J.; Torio, M.; Recuenco, M. Potential Antihypertensive Activities from the Peptic and A-Chymotryptic Hydrolysates of Purified 7S Globulins of Hyacinth Bean (Lablab purpureus L.). In Proceedings of the 44th Annual Convention of the Kapisanang Kimika ng Pilipinas—Southern Tagalog Chapter, B.P. International—Makiling, Jamboree Site, University of the Philippines Los Baños, College, Laguna, Philippines, 5–6 November 2015; p. 12. [Google Scholar]
- Viernes, L.; Garcia, R.; Torio, M.; Garcia, R. Antihypertensive Peptides from Vicilin, the Major Storage Protein of Mung Bean (Vigna radiata (L.) R. Wilczek). J. Biol. Sci. 2012, 12, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Gomes, M.J.; Pagan, L.U.; Okoshi, M.P. Non-Pharmacological Treatment of Cardiovascular Disease | Importance of Physical Exercise. Arq. Bras. Cardiol. 2019, 113, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Alissa, E.M.; Ferns, G.A. Functional Foods and Nutraceuticals in the Primary Prevention of Cardiovascular Diseases. J. Nutr. Metab. 2012, 2012, 569486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric Assay and Properties of the Angiotensin-Converting Enzyme of Rabbit Lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Karami, Z.; Akbari-Adergani, B. Bioactive Food Derived Peptides: A Review on Correlation between Structure of Bioactive Peptides and Their Functional Properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Doyama, N.; Maruyama, N.; Utsumi, S.; Yoshikawa, M. Introduction of DPR, an Enterostatin Fragment Peptide, into Soybean β-Conglycinin α′ Subunit by Site-Directed Mutagenesis. Biosci. Biotechnol. Biochem. 2004, 68, 253–256. [Google Scholar] [CrossRef] [Green Version]
- Pak, V.V.; Koo, M.; Lee, N.; Kim, M.S.; Kwon, D.Y. Structure-Activity Relationships of the Peptide Ile-Ala-Val-Pro and Its Derivatives Revealed Using the Semi-Empirical AM1 Method. Chem. Nat. Compd. 2005, 41, 454–460. [Google Scholar] [CrossRef]
- Pak, V.V.; Koo, M.; Kwon, D.Y.; Yun, L. Design of a Highly Potent Inhibitory Peptide Acting as a Competitive Inhibitor of HMG-CoA Reductase. Amino Acids 2012, 43, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Azani, N.; Babineau, M.; Bailey, C.D.; Banks, H.; Barbosa, A.R.; Pinto, R.B.; Boatwright, J.S.; Borges, L.M.; Brown, G.K.; Bruneau, A.; et al. (Legume Phylogeny Working Group) A New Subfamily Classification of the Leguminosae Based on a Taxonomically Comprehensive Phylogeny: The Legume Phylogeny Working Group (LPWG). Taxon 2017, 66, 44–77. [Google Scholar] [CrossRef] [Green Version]
- Yahara, T.; Javadi, F.; Onoda, Y.; de Queiroz, L.P.; Faith, D.P.; Prado, D.E.; Akasaka, M.; Kadoya, T.; Ishihama, F.; Davies, S.; et al. Global Legume Diversity Assessment: Concepts, Key Indicators, and Strategies. Taxon 2013, 62, 249–266. [Google Scholar] [CrossRef]
- Cardoso, D.; de Queiroz, L.P.; Pennington, R.T.; de Lima, H.C.; Fonty, É.; Wojciechowski, M.F.; Lavin, M. Revisiting the Phylogeny of Papilionoid Legumes: New Insights from Comprehensively Sampled Early-Branching Lineages. Am. J. Bot. 2012, 99, 1991–2013. [Google Scholar] [CrossRef]
- Sprent, J. Global distribution of legumes. In Legume. Nodulation: A Global Perspective; Sprent, J.I., Ed.; John Wiley & Sons Ltd.: West Sussex, UK, 2009; pp. 35–50. ISBN 978-1-4051-8175-4. [Google Scholar]
- Shuster-Gajzágó, I. Nutritional aspects of legumes. In Cultivated. Plants, Primarily as Food Sources; Fuleky, G., Ed.; Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers: Paris, France, 2009; Volume 1, ISBN 1-84826-100-4. [Google Scholar]
- Bastianelli, D.; Grosjean, F.; Peyronnet, C.; Duparque, M.; Régnier, J.M. Feeding Value of Pea (Pisum sativum L.) 1. Chemical Composition of Different Categories of Pea. Anim. Sci. 1998, 67, 609–619. [Google Scholar] [CrossRef]
- Hove, E.L. Composition and Protein Quality of Sweet Lupin Seed. J. Sci. Food Agric. 1974, 25, 851–859. [Google Scholar] [CrossRef]
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional Quality of Legumes, and Their Role in Cardiometabolic Risk Prevention: A Review. J. Med. Food 2013, 16, 185–198. [Google Scholar] [CrossRef]
- Cruz, F.; de Almeida, H.; dos Santos, D. Growth, Nutritional Status and Nitrogen Metabolism in Vigna unguiculata (L.) Walp. Is Affected by Aluminum. Aust. J. Crop. Sci. 2014, 8, 1132–1139. [Google Scholar]
- Adamu, A.; Ajayi, M.; Oyetunde, J. Inorganic and Proximate Nutritional Composition of Common Beans in Nigeria. EJPAC 2016, 3, 25–28. [Google Scholar]
- Caprioli, G.; Giusti, F.; Ballini, R.; Sagratini, G.; Vila-Donat, P.; Vittori, S.; Fiorini, D. Lipid Nutritional Value of Legumes: Evaluation of Different Extraction Methods and Determination of Fatty Acid Composition. Food Chem. 2016, 192, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Rincón, F.; Martínez, B.; Ibáñez, M.V. Proximate Composition and Antinutritive Substances in Chickpea (Cicer arietinum L.) as Affected by the Biotype Factor. J. Sci. Food Agric. 1998, 78, 382–388. [Google Scholar] [CrossRef]
- Marioli Nobile, C.; Carreras, J.; Grosso, R.; Inga, M.; Silva, M.; Aguilar, R.; Allende, M.; Badini, R.; Martinez, M. Proximate Composition and Seed Lipid Components of “Kabuli”-Type Chickpea (Cicer arietinum L.) from Argentina. Agric. Sci. 2013, 4, 729–737. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, A.; Ateeq, N.; Khalil, I.A.; Perveen, S.; Saleemullah, S. Physicochemical Characteristics and Amino Acid Profile of Chickpea Cultivars Grown in Pakistan. J. Foodserv. 2006, 17, 94–101. [Google Scholar] [CrossRef]
- Nwadike, C.; Okere, A.; Nwosu, D.; Okoye, C.; Vange, T.; Apuyor, B. Proximate and Nutrient Composition of Some Common Bean (Phaseolus vulgaris L.) and Cowpea (Vigna unguiculata L. Walp.) Accessions of Jos-Plateau, Nigeria. J. Agric. Ecol. 2018, 15, 1–9. [Google Scholar] [CrossRef]
- Antova, G.A.; Stoilova, T.D.; Ivanova, M.M. Proximate and Lipid Composition of Cowpea (Vigna unguiculata L.) Cultivated in Bulgaria. J. Food Compos. Anal. 2014, 33, 146–152. [Google Scholar] [CrossRef]
- Punia, K.P. Darshan Proximate Composition, Phytic Acid, Polyphenols and Digestibility (In Vitro) of Four Brown Cowpea Varieties. Int. J. Food Sci. Nutr. 2000, 51, 189–193. [Google Scholar] [CrossRef]
- Henshaw, F. Varietal Differences in Physical Characteristics and Proximate Composition of Cowpea (Vigna unguiculata). WJAS 2008, 4, 302–306. [Google Scholar]
- Hacıseferoǧulları, H.; Gezer, İ.; Bahtiyarca, Y.; Mengeş, H.O. Determination of Some Chemical and Physical Properties of Sakız Faba Bean (Vicia faba L. var. Major). J. Food Eng. 2003, 60, 475–479. [Google Scholar] [CrossRef]
- Grela, E.R.; Günter, K.D. Fatty Acid Composition and Tocopherol Content of Some Legume Seeds. Anim. Feed. Sci. Technol. 1995, 52, 325–331. [Google Scholar] [CrossRef]
- Millar, K.A.; Gallagher, E.; Burke, R.; McCarthy, S.; Barry-Ryan, C. Proximate Composition and Anti-Nutritional Factors of Fava-Bean (Vicia faba), Green-Pea and Yellow-Pea (Pisum sativum) Flour. J. Food Compost. Anal. 2019, 82, 103233. [Google Scholar] [CrossRef]
- Hossain, S.; Ahmed, R.; Bhowmick, S.; Mamun, A.A.; Hashimoto, M. Proximate Composition and Fatty Acid Analysis of Lablab purpureus (L.) Legume Seed: Implicates to Both Protein and Essential Fatty Acid Supplementation. Springerplus 2016, 5, 1899. [Google Scholar] [CrossRef] [Green Version]
- Habib, H.M.; Theuri, S.W.; Kheadr, E.E.; Mohamed, F.E. Functional, Bioactive, Biochemical, and Physicochemical Properties of the Dolichos lablab Bean. Food Funct. 2017, 8, 872–880. [Google Scholar] [CrossRef]
- Vadivel, V.; Janardhanan, K. Diversity in Nutritional Composition of Wild Jack Bean (Canavalia ensiformis L. DC) Seeds Collected from South India. Food Chem. 2001, 74, 507–511. [Google Scholar] [CrossRef]
- Abitogun, A.; Olasehinde, E. Nutritional Evaluation of Seed and Characterization of Crude Jack Bean (Canavalia ensiformis) Oil. IOSR-JAC 2012, 1, 36–40. [Google Scholar] [CrossRef]
- Ramdath, D.D.; Padhi, E.M.T.; Sarfaraz, S.; Renwick, S.; Duncan, A.M. Beyond the Cholesterol-Lowering Effect of Soy Protein: A Review of the Effects of Dietary Soy and Its Constituents on Risk Factors for Cardiovascular Disease. Nutrients 2017, 9, 324. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, I.M.M.S. Effects of Water Stress on the Proximate Composition and Mineral Contents of Seeds of Two Lupins (Lupinus albus and Lupinus mutabilis). J. Food Qual. 2005, 28, 325–332. [Google Scholar] [CrossRef]
- Garcıa-López, P.M.; Muzquiz, M.; Ruiz-Lopez, M.A.; Zamora-Natera, J.F.; Burbano, C.; Pedrosa, M.M.; Cuadrado, C.; Garzón-De la Mora, P. Chemical Composition and Fatty Acid Profile of Several Mexican Wild Lupins. J. Food Compost. Anal. 2001, 14, 645–651. [Google Scholar] [CrossRef]
- Musco, N.; Cutrignelli, M.I.; Calabrò, S.; Tudisco, R.; Infascelli, F.; Grazioli, R.; Lo Presti, V.; Gresta, F.; Chiofalo, B. Comparison of Nutritional and Antinutritional Traits among Different Species (Lupinus albus L., Lupinus luteus L., Lupinus angustifolius L.) and Varieties of Lupin Seeds. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1227–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opara, C.; Egbuonu, A.; Obike, C. Assessment of Proximate, Vitamins, Minerals and Anti-Nutrients Compositions of Unprocessed Vigna aconitifolia (Moth Bean) Seeds. ACRI 2017, 11, 1–7. [Google Scholar] [CrossRef]
- Shet, M.S.; Murugiswamy, B.; Madaiah, M. Lipid Profile and Fatty Acid Composition of Moth Bean (Vigna aconitefolia) Seeds. Fette Seifen Anstrichm. 1986, 88, 264–266. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Przybylski, R.; Sultana, B.; Ashraf, M. Chemical Composition and Antioxidant Activity of Seeds of Different Cultivars of Mungbean. J. Food Sci. 2007, 72, S503–S510. [Google Scholar] [CrossRef]
- El-Adawy, T.A.; Rahma, E.H.; El-Bedawey, A.A.; El-Beltagy, A.E. Nutritional Potential and Functional Properties of Germinated Mung Bean, Pea and Lentil Seeds. Plant Foods Hum. Nutr. 2003, 58, 1–13. [Google Scholar] [CrossRef]
- Abdel-Rahman, E.-S.A.; El-Fishawy, F.A.; El-Geddawy, M.A.; Kurz, T.; El-Rify, M.N. The Changes in the Lipid Composition of Mung Bean Seeds as Affected by Processing Methods. Int. J. Food Eng. 2007, 3. [Google Scholar] [CrossRef]
- Hove, E.L.; King, S.; Hill, G.D. Composition, Protein Quality, and Toxins of Seeds of the Grain Legumes Glycine max, Lupinus spp., Phaseolus spp., Pisum sativum, and Vicia faba. N. Z. J. Agric. Res. 1978, 21, 457–462. [Google Scholar] [CrossRef] [Green Version]
- Atasie, V.; Akinhanmi, T.; Ojiodu, C. Proximate Analysis and Physico-Chemical Properties of Groundnut (Arachis hypogaea L.). Pak. J. Nut. 2009, 8, 194–197. [Google Scholar] [CrossRef]
- Etiosa, O.; Chika, N.; Benedicta, A. Mineral and Proximate Composition of Soya Bean. Asian J. Phys. Chem. Sci. 2017, 4, 1–6. [Google Scholar] [CrossRef]
- Padgette, S.R.; Taylor, N.B.; Nida, D.L.; Bailey, M.R.; MacDonald, J.; Holden, L.R.; Fuchs, R.L. The Composition of Glyphosate-Tolerant Soybean Seeds Is Equivalent to That of Conventional Soybeans. J. Nutr. 1996, 126, 702–716. [Google Scholar] [CrossRef] [Green Version]
- Fehr, W.R. Breeding for Modified Fatty Acid Composition in Soybean. Crop. Sci. 2007, 47, S-72. [Google Scholar] [CrossRef]
- Bazzano, L.; Tees, M.T.; Nguyen, C.H. Effect of Non-Soy Legume Consumption on Cholesterol Levels: A Meta-Analysis of Randomized Controlled Trial. Circulation 2008, 118, S1122. [Google Scholar]
- Wang, H.X.; Ng, T.B. An Antifungal Peptide from Red Lentil Seeds. Peptides 2007, 28, 547–552. [Google Scholar] [CrossRef]
- Malaguti, M.; Dinelli, G.; Leoncini, E.; Bregola, V.; Bosi, S.; Cicero, A.; Hrelia, S. Bioactive Peptides in Cereals and Legumes: Agronomical, Biochemical and Clinical Aspects. Int. J. Mol. Sci. 2014, 15, 21120–21135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayathilake, C.; Visvanathan, R.; Deen, A.; Bangamuwage, R.; Jayawardana, B.C.; Nammi, S.; Liyanage, R. Cowpea: An Overview on Its Nutritional Facts and Health Benefits. J. Sci. Food Agric. 2018, 98, 4793–4806. [Google Scholar] [CrossRef] [PubMed]
- Rizkalla, S.W.; Bellisle, F.; Slama, G. Health Benefits of Low Glycaemic Index Foods, Such as Pulses, in Diabetic Patients and Healthy Individuals. Br. J. Nutr. 2002, 88, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Serventi, L.; Dsouza, L. Bioactives in legumes. In Upcycling Legume Water: From Wastewater to Food Ingredients; Springer: Cham, Switzerland, 2020; pp. 139–153. ISBN 978-3-030-42468-8. [Google Scholar]
- Moreno-Valdespino, C.A.; Luna-Vital, D.; Camacho-Ruiz, R.M.; Mojica, L. Bioactive Proteins and Phytochemicals from Legumes: Mechanisms of Action Preventing Obesity and Type-2 Diabetes. Food Res. Int. 2019, 108905. [Google Scholar] [CrossRef]
- Moghaddam, A.S.; Entezari, M.H.; Iraj, B.; Askari, G.R. The Effects of Consumption of Bread Fortified With Soy Bean Flour on Metabolic Profile in Type 2 Diabetic Women: A Cross-over Randomized Controlled Clinical Trial. Int. J. Prev. Med. 2014, 5, 1529–1536. [Google Scholar] [PubMed]
- Garg, S.; Lule, V.K.; Malik, R.K. Soy Bioactive Components in Functional Perspective: A Review. Int. J. Food Prop. 2016, 19, 2550–2574. [Google Scholar] [CrossRef] [Green Version]
- Si, H.; Liu, D. Genistein, a Soy Phytoestrogen, Up-Regulates the Expression of Human Endothelial Nitric Oxide Synthase and Lowers Blood Pressure in Spontaneously Hypertensive Rats. J. Nutr. 2008, 138, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Jiang, W.; Thompson, H.J. Edible Dry Bean Consumption (Phaseolus vulgaris L.) Modulates Cardiovascular Risk Factors and Diet-Induced Obesity in Rats and Mice. Br. J. Nutr. 2012, 108, S66–S73. [Google Scholar] [CrossRef] [Green Version]
- Thompson, H.J.; McGinley, J.N.; Neil, E.S.; Brick, M.A. Beneficial Effects of Common Bean on Adiposity and Lipid Metabolism. Nutrients 2017, 9, 998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abeysekara, S.; Chilibeck, P.D.; Vatanparast, H.; Zello, G.A. A Pulse-Based Diet Is Effective for Reducing Total and LDL-Cholesterol in Older Adults. Br. J. Nutr. 2012, 108, S103–S110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winham, D.M.; Hutchins, A.M. Baked Bean Consumption Reduces Serum Cholesterol in Hypercholesterolemic Adults. Nutr. Res. 2007, 27, 380–386. [Google Scholar] [CrossRef]
- Kabagambe, E.K.; Baylin, A.; Ruiz-Narvarez, E.; Siles, X.; Campos, H. Decreased Consumption of Dried Mature Beans Is Positively Associated with Urbanization and Nonfatal Acute Myocardial Infarction. J. Nutr. 2005, 135, 1770–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flight, I.; Clifton, P. Cereal Grains and Legumes in the Prevention of Coronary Heart Disease and Stroke: A Review of the Literature. Eur. J. Clin. Nutr. 2006, 60, 1145–1159. [Google Scholar] [CrossRef] [Green Version]
- Pennacchio, L.A.; Olivier, M.; Hubacek, J.A.; Cohen, J.-C.; Cox, D.R.; Fruchart, J.C.; Krauss, R.M.; Rubin, E.M. An Apolipoprotein Influencing Triglycerides in Humans and Mice Revealed by Comparative Sequencing. Science 2001, 294, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plump, A.S.; Smith, J.D.; Hayek, T.; Aalto-Setälä, K.; Walsh, A.; Verstuyft, J.G.; Rubin, E.M.; Breslow, J.L. Severe Hypercholesterolemia and Atherosclerosis in Apolipoprotein E-Deficient Mice Created by Homologous Recombination in ES Cells. Cell 1992, 71, 343–353. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, J.Y.; Kim, O.Y.; Lee, J.E.; Cho, H.; Ordovas, J.M.; Lee, J.H. The −1131T→C Polymorphism in the Apolipoprotein A5 Gene Is Associated with Postprandial Hypertriacylglycerolemia; Elevated Small, Dense LDL Concentrations; and Oxidative Stress in Non-Obese Korean Men. Am. J. Clin. Nutr. 2004, 80, 832–840. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.; Paik, J.K.; Hyun, Y.J.; Chae, J.S.; Kim, J.Y.; Choi, J.R.; Lee, S.H.; Shin, D.-J.; Ordovas, J.M.; Lee, J.H. The Apolipoprotein A5 -1131T>C Promoter Polymorphism in Koreans: Association with Plasma APOA5 and Serum Triglyceride Concentrations, LDL Particle Size and Coronary Artery Disease. Clin. Chim. Acta 2009, 402, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Talmud, P.J.; Martin, S.; Taskinen, M.-R.; Frick, M.H.; Nieminen, M.S.; Kesäniemi, Y.A.; Pasternarck, A.; Humphries, S.E.; Syvänne, M. APOA5 Gene Variants, Lipoprotein Particle Distribution, and Progression of Coronary Heart Disease: Results from the LOCAT Study. J. Lipid. Res. 2004, 45, 750–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabika, T.; Nasreen, S.; Kobayashi, S.; Masuda, J. The Genetic Effect of the Apoprotein AV Gene on the Serum Triglyceride Level in Japanese. Atherosclerosis 2002, 165, 201–204. [Google Scholar] [CrossRef]
- Charriere, S.; Bernard, S.; Aqallal, M.; Merlin, M.; Billon, S.; Perrot, L.; Le Coquil, E.; Sassolas, A.; Moulin, P.; Marcais, C. Association of APOA5 −1131T>C and S19W Gene Polymorphisms with Both Mild Hypertriglyceridemia and Hyperchylomicronemia in Type 2 Diabetic Patients. Clin. Chim. Acta 2008, 394, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Chae, J.S.; Kim, O.Y.; Park, H.J.; Kim, J.Y.; Paik, J.K.; Lee, S.-H.; Lee, J.H. APOA5-1131T>C Genotype Effects on Apolipoprotein A5 and Triglyceride Levels in Response to Dietary Intervention and Regular Exercise (DIRE) in Hypertriglyceridemic Subjects. Atherosclerosis 2010, 211, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Kim, M.; Chae, J.S.; Lee, S.-H.; Lee, J.H. Consumption of Whole Grains and Legumes Modulates the Genetic Effect of the APOA5 -1131C Variant on Changes in Triglyceride and Apolipoprotein A-V Concentrations in Patients with Impaired Fasting Glucose or Newly Diagnosed Type 2 Diabetes. Trials 2014, 15, 100. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, E.S.; Amaral, A.L.S.; Demonte, A.; Zanelli, C.F.; Capraro, J.; Duranti, M.; Neves, V.A. Hypocholesterolaemic Effect of Rat-Administered Oral Doses of the Isolated 7S Globulins from Cowpeas and Adzuki Beans. J. Nutr. Sci. 2015, 4, e7. [Google Scholar] [CrossRef] [Green Version]
- Washburn, S.; Burke, G.; Morgan, T.; Anthony, M. Effect of Soy Protein Supplementation on Serum Lipoproteins, Blood Pressure, and Menopausal Symptoms in Perimenopausal Women. Menopause 1999, 6, 7–13. [Google Scholar] [CrossRef]
- Burke, V.; Hodgson, J.; Beilin, L.; Giangiulioi, N.; Rogers, P.; Puddey, I. Dietary Protein and Soluble Fiber Reduce Ambulatory Blood Pressure in Treated Hypertensives. Hypertension 2001, 38, 821–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darmadi-Blackberry, I.; Wahlqvist, M.L.; Kouris-Blazos, A.; Steen, B.; Lukito, W.; Horie, Y.; Horie, K. Legumes: The Most Important Dietary Predictor of Survival in Older People of Different Ethnicities. Asia Pac. J. Clin. Nutr. 2004, 13, 217–220. [Google Scholar] [PubMed]
- He, J.; Gu, D.; Wu, X.; Chen, J.; Duan, X.; Chen, J.; Whelton, P.K. Effect of Soybean Protein on Blood Pressure: A Randomized, Controlled Trial. Ann. Intern. Med. 2005, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.; Maltais, A. Pulses a Novel Protein Source. Agro Food Ind. Hi Tech 2011, 22, 24–26. [Google Scholar]
- Sirtori, C.R.; Lovati, M.R.; Manzoni, C.; Monetti, M.; Pazzucconi, F.; Gatti, E. Soy and Cholesterol Reduction: Clinical Experience. J. Nutr. 1995, 125, 598S–605S. [Google Scholar] [CrossRef]
- Madani, S.; Prost, J.; Narce, M.; Belleville, J. VLDL Metabolism in Rats Is Affected by the Concentration and Source of Dietary Protein. J. Nutr. 2003, 133, 4102–4106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.W.; Major, A.W. Pulses and Lipaemia, Short- and Long-Term Effect: Potential in the Prevention of Cardiovascular Disease. Br. J. Nutr. 2002, 88, 263–271. [Google Scholar] [CrossRef]
- Reynolds, K.; Chin, A.; Lees, K.A.; Nguyen, A.; Bujnowski, D.; He, J. A Meta-Analysis of the Effect of Soy Protein Supplementation on Serum Lipids. Am. J. Cardiol. 2006, 98, 633–640. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Thompson, A.M.; Tees, M.T.; Nguyen, C.H.; Winham, D.M. Non-Soy Legume Consumption Lowers Cholesterol Levels: A Meta-Analysis of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Belski, R. Chapter 4—Fiber, protein, and lupin-enriched foods: Role for improving cardiovascular health. In Advances in Food and Nutrition Research; Henry, J., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 66, pp. 147–215. ISBN 1043-4526. [Google Scholar]
- Hayat, I.; Ahmad, A.; Masud, T.; Ahmed, A.; Bashir, S. Nutritional and Health Perspectives of Beans (Phaseolus vulgaris L.): An Overview. Crit. Rev. Food Sci. Nutr. 2014, 54, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Tovar, A.R.; Murguía, F.; Cruz, C.; Hernández-Pando, R.; Aguilar-Salinas, C.A.; Pedraza-Chaverri, J.; Correa-Rotter, R.; Torres, N. A Soy Protein Diet Alters Hepatic Lipid Metabolism Gene Expression and Reduces Serum Lipids and Renal Fibrogenic Cytokines in Rats with Chronic Nephrotic Syndrome. J. Nutr. 2002, 132, 2562–2569. [Google Scholar] [CrossRef]
- Sirtori, C.; Galli, G.; Lovati, M.; Carrara, P.; Bosisio, E.; Kienle, M. Effects of Dietary Proteins on the Regulation of Liver Lipoprotein Receptors in Rats. J. Nutr. 1984, 114, 1493–1500. [Google Scholar] [CrossRef] [Green Version]
- Vu-Dac, N.; Gervois, P.; Torra, I.P.; Fruchart, J.C.; Kosykh, V.; Kooistra, T.; Princen, H.M.; Dallongeville, J.; Staels, B. Retinoids Increase Human Apo C-III Expression at the Transcriptional Level via the Retinoid X Receptor. Contribution to the Hypertriglyceridemic Action of Retinoids. J. Clin. Investig. 1998, 102, 625–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, C.; Mei, J.; Wood, C. Effect of Soy Proteins and Isoflavones on Lipid Metabolism and Involved Gene Expression. Front. Biosci. 2008, 13, 2660–2673. [Google Scholar] [CrossRef] [Green Version]
- Xiao, C.W.; Mei, J.; Huang, W.; Wood, C.; L’Abbé, M.R.; Gilani, G.S.; Cooke, G.M.; Curran, I.H. Dietary Soy Protein Isolate Modifies Hepatic Retinoic Acid Receptor-β Proteins and Inhibits Their DNA Binding Activity in Rats. J. Nutr. 2007, 137, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Seppo, L.; Jauhiainen, T.; Poussa, T.; Korpela, R. A Fermented Milk High in Bioactive Peptides Has a Blood Pressure–Lowering Effect in Hypertensive Subjects. Am. J. Clin. Nutr. 2003, 77, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.H.; Ko, J.H.; Ahn, C.W.; Lee, H.H.; Shin, J.K.; Chang, S.J.; Park, C.S.; Kang, J.H. In Vivo and in Vitro Application of Black Soybean Peptides in the Amelioration of Endoplasmic Reticulum Stress and Improvement of Insulin Resistance. Life Sci. 2010, 86, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Hodges, R.E.; Krehl, W.A.; Stone, D.B.; Lopez, A. Dietary Carbohydrates and Low Cholesterol Diets: Effects on Serum Lipids of Man. Am. J. Clin. Nutr. 1967, 20, 198–208. [Google Scholar] [CrossRef]
- Lyn-Cook, B.D.; Blann, E.; Payne, P.W.; Bo, J.; Sheehan, D.; Medlock, K. Methylation Profile and Amplification of Proto-Oncogenes in Rat Pancreas Induced with Phytoestrogens. Proc. Soc. Exp. Biol. Med. 1995, 208, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Johnstone, B.M.; Cook-Newell, M.E. Meta-Analysis of the Effects of Soy Protein Intake on Serum Lipids. N. Engl. J. Med. 1995, 333, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.; Galli, C.; Anderson, J.; Sirtori, E.; Arnoldi, A. Functional Foods for Dyslipidaemia and Cardiovascular Risk Prevention. Nutr. Res. Rev. 2009, 22, 244–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, D.J.A.; Mirrahimi, A.; Srichaikul, K.; Berryman, C.E.; Wang, L.; Carleton, A.; Abdulnour, S.; Sievenpiper, J.L.; Kendall, C.W.C.; Kris-Etherton, P.M. Soy Protein Reduces Serum Cholesterol by Both Intrinsic and Food Displacement Mechanisms. J. Nutr. 2010, 140, 2302S–2311S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsythe, W.A., III. Comparison of Dietary Casein or Soy Protein Effects on Plasma Lipids and Hormone Concentrations in the Gerbil (Meriones unguiculatus). J. Nutr. 1986, 116, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Sugano, M. Effects of Addition of Sulfur-Containing Amino Acids and Glycine to Soybean Protein and Casein on Serum Cholesterol Levels of Rats. J. Nutr. Sci. Vitaminol. 1989, 35, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Nagata, C.; Takatsuka, N.; Kurisu, Y.; Shimizu, H. Decreased Serum Total Cholesterol Concentration Is Associated with High Intake of Soy Products in Japanese Men and Women. J. Nutr. 1998, 128, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Woo, J.L.F.; Leung, S.S.F.; Sham, A.L.K.; Lam, T.H.; Janus, E.D. Intake of Soy Products Is Associated with Better Plasma Lipid Profiles in the Hong Kong Chinese Population. J. Nutr. 2000, 130, 2590–2593. [Google Scholar] [CrossRef] [Green Version]
- Lovati, M.R.; Manzoni, C.; Gianazza, E.; Arnoldi, A.; Kurowska, E.; Carroll, K.K.; Sirtori, C.R. Soy Protein Peptides Regulate Cholesterol Homeostasis in Hep G2 Cells. J. Nutr. 2000, 130, 2543–2549. [Google Scholar] [CrossRef] [Green Version]
- Imai, S. Soybean and Processed Soy Foods Ingredients, and Their Role in Cardiometabolic Risk Prevention. Recent Pat. Food Nutr. Agric. 2015, 7, 75–82. [Google Scholar] [CrossRef]
- Sugano, M.; Goto, S.; Yamada, Y.; Yoshida, K.; Hashimoto, Y.; Matsuo, T.; Kimoto, M. Cholesterol-Lowering Activity of Various Undigested Fractions of Soybean Protein in Rats. J. Nutr. 1990, 120, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-F.; Yamamoto, S.; Chung, H.-M.; Chung, S.-Y.; Miyatani, S.; Mori, M.; Okita, T.; Sugano, M. Antihypercholesterolemic Effect of Undigested Fraction of Soybean Protein in Young Female Volunteers. J. Nutr. Sci. Vitaminol. 1995, 41, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Mackay, S.; Ball, M. Do Beans and Oat Bran Add to the Effectiveness of a Low-Fat Diet? Eur. J. Clin. Nutr. 1992, 46, 641–648. [Google Scholar] [PubMed]
- Chen, H.; Muramoto, K.; Yamauchi, F. Structural Analysis of Antioxidative Peptides from Soybean β-Conglycinin. J. Agric. Food Chem. 1995, 43, 574–578. [Google Scholar] [CrossRef]
- Wade, A.M.; Tucker, H.N. Antioxidant Characteristics of L-Histidine. J. Nutr. Biochem. 1998, 9, 308–315. [Google Scholar] [CrossRef]
- Beermann, C.; Euler, M.; Herzberg, J.; Stahl, B. Anti-Oxidative Capacity of Enzymatically Released Peptides from Soybean Protein Isolate. Eur. Food Res. Technol. 2009, 229, 637–644. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.; Baek, H.; Lee, H. Purification and Characterization of Antioxidant Peptides from Soy Protein Hydrolysate. J. Food Biochem. 2010, 34, 120–132. [Google Scholar] [CrossRef]
- Chen, H.-M.; Muramoto, K.; Yamauchi, F.; Nokihara, K. Antioxidant Activity of Designed Peptides Based on the Antioxidative Peptide Isolated from Digests of a Soybean Protein. J. Agric. Food Chem. 1996, 44, 2619–2623. [Google Scholar] [CrossRef]
- Xiao, C.; Wood, C.; Huang, W.; Abbé, M.; Gilani, G.; Gerard, M.; Curran, I. Tissue-Specific Regulation of Acetyl-CoA Carboxylase Gene Expression by Dietary Soya Protein Isolate in Rats. Br. J. Nutr. 2006, 95, 1048–1054. [Google Scholar] [CrossRef]
- Sugano, M.; Yamada, Y.; Yoshida, K.; Hashimoto, Y.; Matsuo, T.; Kimoto, M. The Hypocholesterolemic Action of the Undigested Fraction of Soybean Protein in Rats. Atherosclerosis 1988, 72, 115. [Google Scholar] [CrossRef]
- Huff, M.; Carroll, K. Effects of Dietary Protein on Turnover, Oxidation, and Absorption of Cholesterol, and on Steroid Excretion in Rabbits. J. Lipid Res. 1980, 21, 546–548. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S.; Hati, S. Functional Significance of Bioactive Peptides Derived from Soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef]
- Anthony, M.; Clarkson, T.; Bullock, B.; Wagner, J. Soy Protein versus Soy Phytoestrogens in the Prevention of Diet-Induced Coronary Artery Atherosclerosis of Male Cynomolgus Monkeys. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.L.; Vafeiadou, K.; Hallund, J.; Bugel, S.; Reimann, M.; Koebnick, C.; Zunft, H.J.F.; Ferrari, M.; Branca, F.; Dadd, T.; et al. Soy-Isoflavone-Enriched Foods and Markers of Lipid and Glucose Metabolism in Postmenopausal Women: Interactions with Genotype and Equol Production. Am. J. Clin. Nutr. 2006, 83, 592–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibbs, B.F.; Zougman, A.; Masse, R.; Mulligan, C. Production and Characterization of Bioactive Peptides from Soy Hydrolysate and Soy-Fermented Food. Food Res. Int. 2004, 37, 123–131. [Google Scholar] [CrossRef]
- Ferreira, E.D.S.; Silva, M.A.; Demonte, A.; Neves, V.A. β-Conglycinin (7S) and Glycinin (11S) Exert a Hypocholesterolemic Effect Comparable to That of Fenofibrate in Rats Fed a High-Cholesterol Diet. J. Funct. Foods 2010, 2, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, E.D.S.; Silva, M.A.; Demonte, A.; Neves, V.A. Soy β-Conglycinin (7S Globulin) Reduces Plasma and Liver Cholesterol in Rats Fed Hypercholesterolemic Diet. J. Med. Food 2010, 14, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Duranti, M.; Lovati, M.R.; Dani, V.; Barbiroli, A.; Scarafoni, A.; Castiglioni, S.; Ponzone, C.; Morazzoni, P. The α′ Subunit from Soybean 7S Globulin Lowers Plasma Lipids and Upregulates Liver β-VLDL Receptors in Rats Fed a Hypercholesterolemic Diet. J. Nutr. 2004, 134, 1334–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohno, M.; Hirotsuka, M.; Kito, M.; Matsuzawa, Y. Decreases in Serum Triacylglycerol and Visceral Fat Mediated by Dietary Soybean β-Conglycinin. J. Atheroscler. Thromb. 2006, 13, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Scarafoni, A.; Magni, C.; Duranti, M. Molecular Nutraceutics as a Mean to Investigate the Positive Effects of Legume Seed Proteins on Human Health. Trends Food Sci. Technol. 2007, 18, 454–463. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Bringe, N.A.; Berhow, M.A.; Gonzalez de Mejia, E. β-Conglycinin Embeds Active Peptides That Inhibit Lipid Accumulation in 3T3-L1 Adipocytes in Vitro. J. Agric. Food Chem. 2008, 56, 10533–10543. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Bringe, N.A.; Berhow, M.A.; Gonzalez de Mejia, E. β-Conglycinins among Sources of Bioactives in Hydrolysates of Different Soybean Varieties That Inhibit Leukemia Cells in Vitro. J. Agric. Food Chem. 2008, 56, 4012–4020. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Villaluenga, C.; Dia, V.P.; Berhow, M.; Bringe, N.A.; Gonzalez de Mejia, E. Protein Hydrolysates from β-Conglycinin Enriched Soybean Genotypes Inhibit Lipid Accumulation and Inflammation in Vitro. Mol. Nutr. Food Res. 2009, 53, 1007–1018. [Google Scholar] [CrossRef]
- Pak, V.V.; Koo, M.S.; Kasymova, T.D.; Kwon, D.Y. Isolation and Identification of Peptides from Soy 11S-Globulin with Hypocholesterolemic Activity. Chem. Nat. Compd. 2005, 41, 710–714. [Google Scholar] [CrossRef]
- Lovati, M.R.; Manzoni, C.; Corsini, A.; Granata, A.; Frattini, R.; Fumagalli, R.; Sirtori, C.R. Low Density Lipoprotein Receptor Activity Is Modulated by Soybean Globulins in Cell Culture. J. Nutr. 1992, 122, 1971. [Google Scholar] [CrossRef]
- Lovati, M.R.; Manzoni, C.; Corsini, A.; Granata, A.; Fumagalli, R.; Sirtori, C.R. 7S Globulin from Soybean Is Metabolized in Human Cell Cultures by a Specific Uptake and Degradation System. J. Nutr. 1996, 126, 2831. [Google Scholar]
- Mochizuki, Y.; Maebuchi, M.; Kohno, M.; Hirotsuka, M.; Wadahama, H.; Moriyama, T.; Kawada, T.; Urade, R. Changes in Lipid Metabolism by Soy β-Conglycinin-Derived Peptides in HepG2 Cells. J. Agric. Food Chem. 2009, 57, 1473–1480. [Google Scholar] [CrossRef]
- Manzoni, C.; Lovati, M.R.; Gianazza, E.; Morita, Y.; Sirtori, C.R. Soybean Protein Products as Regulators of Liver Low-Density Lipoprotein Receptors. II. A−α‘ Rich Commercial Soy Concentrate and α‘ Deficient Mutant Differently Affect Low-Density Lipoprotein Receptor Activation. J. Agric. Food Chem. 1998, 46, 2481–2484. [Google Scholar] [CrossRef]
- Manzoni, C.; Duranti, M.; Eberini, I.; Scharnag, H.; März, W.; Castiglioni, S.; Lovati, M. Subcellular Localization of Soybean 7S Globulin in HepG2 Cells and LDL Receptor Up-Regulation by Its α′ Constituent Subunit. J. Nutr. 2003, 133, 2149–2155. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, T.; Kishimoto, K.; Nagai, K.; Urade, R.; Ogawa, T.; Utsumi, S.; Maruyama, N.; Maebuchi, M. Soybean Beta-Conglycinin Diet Suppresses Serum Triglyceride Levels in Normal and Genetically Obese Mice by Induction of Beta-Oxidation, Downregulation of Fatty Acid Synthase, and Inhibition of Triglyceride Absorption. Biosci. Biotechnol. Biochem. 2004, 68, 352–359. [Google Scholar] [CrossRef]
- Shimomura, I.; Bashmakov, Y.; Horton, J.D. Increased Levels of Nuclear SREBP-1c Associated with Fatty Livers in Two Mouse Models of Diabetes Mellitus. J. Biol. Chem. 1999, 274, 30028–30032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, A.; Brandsch, C.; Bettzieche, A.; Hirche, F.; Stangl, G.I.; Eder, K. Isoflavone-Poor Soy Protein Alters the Lipid Metabolism of Rats by SREBP-Mediated Down-Regulation of Hepatic Genes. J. Nutr. Biochem. 2007, 18, 313–321. [Google Scholar] [CrossRef]
- Lovati, M.R.; Manzoni, C.; Gianazza, E.; Sirtori, C.R. Soybean Protein Products as Regulators of Liver Low-Density Lipoprotein Receptors. I. Identification of Active β-Conglycinin Subunits. J. Agric. Food Chem. 1998, 46, 2474–2480. [Google Scholar] [CrossRef]
- Galvez, A. Identification of Lunasin as the Active Component in Soy Protein Responsible for Reducing LDL Cholesterol and Risk of Cardiovascular Disease. Circulation 2012, 126, A10693. [Google Scholar] [CrossRef]
- Fontanari, G.G.; Batistuti, J.P.; Da Cruz, R.J.; Saldiva, P.H.N.; Arêas, J.A.G. Cholesterol-Lowering Effect of Whole Lupin (Lupinus albus) Seed and Its Protein Isolate. Food Chem. 2012, 132, 1521–1526. [Google Scholar] [CrossRef] [Green Version]
- Sirtori, C.R.; Lovati, M.R.; Manzoni, C.; Castiglioni, S.; Duranti, M.; Magni, C.; Morandi, S.; D’Agostina, A.; Arnoldi, A. Proteins of White Lupin Seed, a Naturally Isoflavone-Poor Legume, Reduce Cholesterolemia in Rats and Increase LDL Receptor Activity in HepG2 Cells. J. Nutr. 2004, 134, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Bettzieche, A.; Brandsch, C.; Eder, K.; Stangl, G. Lupin Protein Acts Hypocholesterolemic and Increases Milk Fat Content in Lactating Rats by Influencing the Expression of Genes Involved in Cholesterol Homeostasis and Triglyceride Synthesis. Mol. Nutr. Food Res. 2009, 53, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, M.; Parolini, C.; Diani, E.; Rigamonti, E.; Cornelli, L.; Arnoldi, A.; Sirtori, C.R.; Chiesa, G. Hypolipidaemic and Anti-Atherosclerotic Effects of Lupin Proteins in a Rabbit Model. Br. J. Nutr. 2008, 100, 707–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolini, C.; Rigamonti, E.; Marchesi, M.; Busnelli, M.; Cinquanta, P.; Manzini, S.; Sirtori, C.R.; Chiesa, G. Cholesterol-Lowering Effect of Dietary Lupinus angustifolius Proteins in Adult Rats through Regulation of Genes Involved in Cholesterol Homeostasis. Food Chem. 2012, 132, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Bähr, M.; Fechner, A.; Krämer, J.; Kiehntopf, M.; Jahreis, G. Lupin Protein Positively Affects Plasma LDL Cholesterol and LDL:HDL Cholesterol Ratio in Hypercholesterolemic Adults after Four Weeks of Supplementation: A Randomized, Controlled Crossover Study. Nutr. J. 2013, 12, 107. [Google Scholar] [CrossRef] [Green Version]
- Vairappan, B. Chapter 15—Cholesterol Regulation by Leptin in Alcoholic Liver Disease. In Molecular Aspects of Alcohol and Nutrition; Patel, V.B., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 187–200. ISBN 978-0-12-800773-0. [Google Scholar]
- Robinet, P.; Smith, J.D. Chapter 13—Role of Autophagy in Atherogenesis. In Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging; Hayat, M.A., Ed.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 203–211. ISBN 978-0-12-801033-4. [Google Scholar]
- Weiße, K.; Brandsch, C.; Zernsdorf, B.; Nkengfack Nembongwe, G.S.; Hofmann, K.; Eder, K.; Stangl, G.I. Lupin Protein Compared to Casein Lowers the LDL Cholesterol:HDL Cholesterol-Ratio of Hypercholesterolemic Adults. Eur. J. Nutr. 2010, 49, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, C.; Aiello, G.; Arnoldi, A.; Lammi, C. Investigations on the Hypocholesterolaemic Activity of LILPKHSDAD and LTFPGSAED, Two Peptides from Lupin β-Conglutin: Focus on LDLR and PCSK9 Pathways. J. Funct. Foods 2017, 32, 1–8. [Google Scholar] [CrossRef]
- Radtke, J.; Schutkowski, A.; Brandsch, C.; Hirche, F.; Hasenkopf, K.; Stangl, G.I. Isolated Conglutin γ from Lupin, but Not Phytate, Lowers Serum Cholesterol without Influencing Vascular Lesion Development in the ApoE-Deficient Mouse Model. Plant Foods Hum. Nutr. 2015, 70, 113–118. [Google Scholar] [CrossRef]
- Li, H.; Aluko, R.E. Identification and Inhibitory Properties of Multifunctional Peptides from Pea Protein Hydrolysate. J. Agric. Food Chem. 2010, 58, 11471–11476. [Google Scholar] [CrossRef]
- Lasekan, J.B.; Gueth, L.; Khan, S. Influence of Dietary Golden Pea Proteins versus Casein on Plasma and Hepatic Lipids in Rats. Nutr. Res. 1995, 15, 71–84. [Google Scholar] [CrossRef]
- Rigamonti, E.; Parolini, C.; Marchesi, M.; Diani, E.; Brambilla, S.; Sirtori, C.R.; Chiesa, G. Hypolipidemic Effect of Dietary Pea Proteins: Impact on Genes Regulating Hepatic Lipid Metabolism. Mol. Nutr. Food Res. 2010, 54, S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Spielmann, J.; Stangl, G.I.; Eder, K. Dietary Pea Protein Stimulates Bile Acid Excretion and Lowers Hepatic Cholesterol Concentration in Rats. J. Anim. Physiol. Anim. Nutr. 2008, 92, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bhullar, K.S.; Fan, H.; Liao, W.; Qiao, Y.; Su, D.; Wu, J. Regulatory Effects of a Pea-Derived Peptide Leu-Arg-Trp (LRW) on Dysfunction of Rat Aortic Vascular Smooth Muscle Cells against Angiotensin II Stimulation. J. Agric. Food Chem. 2020, 68, 3947–3953. [Google Scholar] [CrossRef] [PubMed]
- Guang, C.; Phillips, R. Angiotensin I-Converting Enzyme Inhibitory Peptides from Hydrolyzed Cowpea Flour. J. Food Agric. Environ. 2005, 10, 55–59. [Google Scholar]
- Segura-Campos, M.R.; Chel Guerrero, L.A.; Betancur Ancona, D.A. Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Activities of Peptide Fractions Extracted by Ultrafiltration of Cowpea Vigna unguiculata Hydrolysates. J. Sci. Food Agric. 2010, 90, 2512–2518. [Google Scholar] [CrossRef]
- Frota, K.M.G.; Mendonça, S.; Saldiva, P.H.N.; Cruz, R.J.; Arêas, J.A.G. Cholesterol-Lowering Properties of Whole Cowpea Seed and Its Protein Isolate in Hamsters. J. Food Sci. 2008, 73, H235–H240. [Google Scholar] [CrossRef] [Green Version]
- Macarulla, M.T.; Medina, C.; Diego, M.A.D.; Chávarri, M.; Zulet, M.Á.; Martínez, J.A.; Nöel-Suberville, C.; Higueret, P.; Portillo, M.P. Effects of the Whole Seed and a Protein Isolate of Faba Bean (Vicia faba) on the Cholesterol Metabolism of Hypercholesterolaemic Rats. Br. J. Nutr. 2001, 85, 607–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayashima, T.; Okazaki, Y.; Katayama, T.; Hori, K. Dietery Lectin Lowers Serum Cholesterol and Raises Fecal Neutral Sterols in Cholesterol-Fed Rats. J. Nutri. Sci. Vitaminol. 2005, 51, 343–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Shi, Y.; Liu, H.; Le, G. Antihypertensive Effect of Alcalase Generated Mung Bean Protein Hydrolysates in Spontaneously Hypertensive Rats. Eur. Food Res. Technol. 2006, 222, 733–736. [Google Scholar] [CrossRef]
- Yao, Y.; Hao, L.; Shi, Z.; Wang, L.; Cheng, X.; Wang, S.; Ren, G. Mung Bean Decreases Plasma Cholesterol by Up-Regulation of CYP7A1. Plant Foods Hum. Nutr. 2014, 69, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhu, Y.; Ren, G. Mung Bean Protein Increases Plasma Cholesterol by Up-Regulation of Hepatic HMG-CoA Reductase, and CYP7A1 in mRNA Levels. J. Food Nutr. Res. 2014, 2, 770–775. [Google Scholar] [CrossRef] [Green Version]
- Nakatani, A.; Li, X.; Miyamoto, J.; Igarashi, M.; Watanabe, H.; Sutou, A.; Watanabe, K.; Motoyama, T.; Tachibana, N.; Kohno, M.; et al. Dietary Mung Bean Protein Reduces High-Fat Diet-Induced Weight Gain by Modulating Host Bile Acid Metabolism in a Gut Microbiota-Dependent Manner. Biochem. Biophys. Res. Commun. 2018, 501, 955–961. [Google Scholar] [CrossRef]
- Kohno, M.; Motoyama, T.; Shigihara, Y.; Sakamoto, M.; Sugano, H. Improvement of Glucose Metabolism via Mung Bean Protein Consumption: A Clinical Trial of GLUCODIATM Isolated Mung Bean Protein in Japan. Funct. Foods Health. Dis. 2017, 7, 115–134. [Google Scholar] [CrossRef] [Green Version]
- Kohno, M.; Sugano, H.; Shigihara, Y.; Shiraishi, Y.; Motoyama, T. Improvement of Glucose and Lipid Metabolism via Mung Bean Protein Consumption: Clinical Trials of GLUCODIATM Isolated Mung Bean Protein in the USA and Canada. J. Nutr. Sci. 2018, 7, e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, W.; Hou, T.; Liu, W.; Guo, D.; He, H. The Hypolipidemic Effects of Peptides Prepared from Cicer arietinum in Ovariectomized Rats and HepG2 Cells. J. Sci. Food Agric. 2019, 99, 576–586. [Google Scholar] [CrossRef]
- Zulet, M.A.; Macarulla, M.T.; Portillo, M.P.; Noel-Suberville, C.; Higueret, P.; Martínez, J.A. Lipid and Glucose Utilization in Hypercholesterolemic Rats Fed a Diet Containing Heated Chickpea (Cicer aretinum L.): A Potential Functional Food. Int. J. Vitam. Nutr. Res. 1999, 69, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Boualga, A.; Prost, J.; Taleb-Senouci, D.; Krouf, D.; Kharoubi, O.; Lamri-Senhadji, M.; Belleville, J.; Bouchenak, M. Purified Chickpea or Lentil Proteins Impair VLDL Metabolism and Lipoprotein Lipase Activity in Epididymal Fat, but Not in Muscle, Compared to Casein, in Growing Rats. Eur. J. Nutr. 2009, 48, 162–169. [Google Scholar] [CrossRef]
- Jansen, H. Hepatic Lipase: Friend or Foe and under What Circumstances? Curr. Atheroscler. Rep. 2004, 6, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International Table of Glycemic Index and Glycemic Load Values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-González, M.Á.; De La Fuente-Arrillaga, C.; Nunez-Cordoba, J.M.; Basterra-Gortari, F.J.; Beunza, J.J.; Vazquez, Z.; Benito, S.; Tortosa, A.; Bes-Rastrollo, M. Adherence to Mediterranean Diet and Risk of Developing Diabetes: Prospective Cohort Study. BMJ 2008, 336, 1348. [Google Scholar] [CrossRef] [Green Version]
- Xue, Z.; Gao, J.; Zhang, Z.; Yu, W.; Wang, H.; Kou, X. Antihyperlipidemic and Antitumor Effects of Chickpea Albumin Hydrolysate. Plant Foods Hum. Nutr. 2012, 67, 393–400. [Google Scholar] [CrossRef]
- Hanson, M.G.; Taylor, C.G.; Wu, Y.; Anderson, H.D.; Zahradka, P. Lentil Consumption Reduces Resistance Artery Remodeling and Restores Arterial Compliance in the Spontaneously Hypertensive Rats. J. Nutr. Biochem. 2016, 37, 30–38. [Google Scholar] [CrossRef]
- Khazaei, H.; Subedi, M.; Nickerson, M.; Martínez-Villaluenga, C.; Frias, J.; Vandenberg, A. Seed Protein of Lentils: Current Status, Progress, and Food Applications. Foods 2019, 8, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakkar, R.; Raju, R.; Sharma, R. Calmodulin-Dependent Cyclic Nucleotide Phosphodiesterase (PDE1). Cell. Mol. Life Sci. 1999, 55, 1164–1186. [Google Scholar] [CrossRef]
- García-Mora, P.; Martín-Martínez, M.; Angeles Bonache, M.; González-Múniz, R.; Peñas, E.; Frias, J.; Martinez-Villaluenga, C. Identification, Functional Gastrointestinal Stability and Molecular Docking Studies of Lentil Peptides with Dual Antioxidant and Angiotensin I Converting Enzyme Inhibitory Activities. Food Chem. 2017, 221, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Akıllıoğlu, H.G.; Karakaya, S. Effects of Heat Treatment and In Vitro Digestion on the Angiotensin Converting Enzyme Inhibitory Activity of Some Legume Species. Eur. Food Res. Techol. 2009, 229, 915–921. [Google Scholar] [CrossRef]
- Barbana, C.; Boye, J.I. Angiotensin I-Converting Enzyme Inhibitory Properties of Lentil Protein Hydrolysates: Determination of the Kinetics of Inhibition. Food Chem. 2011, 127, 94–101. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Baraniak, B. Activities and Sequences of the Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides Obtained from the Digested Lentil (Lens culinaris) Globulins. Int. J. Food Sci. Technol. 2013, 48, 2363–2369. [Google Scholar] [CrossRef]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. ACE-Inhibitory Activity of Enzymatic Protein Hydrolysates from Lupin and Other Legumes. Food Chem. 2014, 145, 34–40. [Google Scholar] [CrossRef]
- Mayilvaganan, M.; Singh, S.; Johari, R. Hypocholesterolemic Effect of Protein Prepared from Phaseolus aconitifolius (Jacq.). Indian J. Exp. Bot. 2004, 42, 904–908. [Google Scholar]
- Potter, S.M. Overview of Proposed Mechanisms for the Hypocholesterolemic Effect of Soy. J. Nutr. 1995, 125, 606S–611S. [Google Scholar] [CrossRef] [PubMed]
- Shin, Z.I.; Yu, R.; Park, S.-A.; Chung, D.K.; Ahn, C.W.; Nam, H.S.; Kim, K.S.; Lee, H.J. His-His-Leu, an Angiotensin I Converting Enzyme Inhibitory Peptide Derived from Korean Soybean Paste, Exerts Antihypertensive Activity in Vivo. J. Agric. Food Chem. 2001, 49, 3004–3009. [Google Scholar] [CrossRef]
- Wu, J.; Ding, X. Hypotensive and Physiological Effect of Angiotensin Converting Enzyme Inhibitory Peptides Derived from Soy Protein on Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2001, 49, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Tachibana, N.; Wanezaki, S.; Tsuzaki, S.; Takamatsu, K.; Yamamoto, T.; Hashimoto, Y.; Shimoda, T. Isoflavone-Free Soy Protein Prepared by Column Chromatography Reduces Plasma Cholesterol in Rats. J. Agric. Food Chem. 2002, 50, 5717. [Google Scholar] [CrossRef]
- Adams, M.R.; Golden, D.L.; Franke, A.A.; Potter, S.M.; Smith, H.S.; Anthony, M.S. Dietary Soy β-Conglycinin (7S Globulin) Inhibits Atherosclerosis in Mice. J. Nutr. 2004, 134, 511–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desroches, S.; Lamarche, B. Diet and Low-Density Lipoprotein Particle Size. Curr. Atheroscler. Rep. 2004, 6, 453–460. [Google Scholar] [CrossRef]
- Zhan, S.; Ho, S.C. Meta-Analysis of the Effects of Soy Protein Containing Isoflavones on the Lipid Profile. Am. J. Clin. Nutr. 2005, 81, 397–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.-Y.; Tong, X.; Wu, Z.-W.; Xun, P.-C.; He, K.; Qin, L.-Q. Effect of Soya Protein on Blood Pressure: A Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2011, 106, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.X.; Li, S.H.; Chen, J.Z.; Sun, K.; Wang, X.J.; Wang, X.G.; Hui, R.T. Effect of Soy Isoflavones on Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 463–470. [Google Scholar] [CrossRef]
- Lavigne, C.; Marette, A.; Jacques, H. Cod and Soy Proteins Compared with Casein Improve Glucose Tolerance and Insulin Sensitivity in Rats. Am. J. Physiol. Endocrinol. 2000, 278, E491–E500. [Google Scholar] [CrossRef] [PubMed]
- Ascencio, C.; Torres, N.; Isoard-Acosta, F.; Gómez-Pérez, F.J.; Hernández-Pando, R.; Tovar, A.R. Soy Protein Affects Serum Insulin and Hepatic SREBP-1 mRNA and Reduces Fatty Liver in Rats. J. Nutr. 2004, 134, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Tovar, A.R.; Torre-Villalvazo, I.; Ochoa, M.; Elías, A.L.; Ortíz, V.; Aguilar-Salinas, C.A.; Torres, N. Soy Protein Reduces Hepatic Lipotoxicity in Hyperinsulinemic Obese Zucker Fa/Fa Rats. J. Lipid Res. 2005, 46, 1823–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, E.S.; Lee, H.H.; Cho, S.Y.; Park, H.W.; Lee, S.J.; Lee, T.R. Genistein Downregulates SREBP-1 Regulated Gene Expression by Inhibiting Site-1 Protease Expression in HepG2 Cells. J. Nutr. 2007, 137, 1127–1131. [Google Scholar] [CrossRef] [Green Version]
- Bayly, G.R. CHAPTER 37—Lipids and disorders of lipoprotein metabolism. In Clinical. Biochemistry: Metabolic and Clinical Aspects, 3rd ed.; Marshall, W.J., Lapsley, M., Day, A.P., Ayling, R.M., Eds.; Churchill Livingstone: London, UK, 2014; pp. 702–736. ISBN 978-0-7020-5140-1. [Google Scholar]
- Robinet, P.; Védie, B.; Chironi, G.; Gariépy, J.; Simon, A.; Moatti, N.; Paul, J.-L. Characterization of Polymorphic Structure of SREBP-2 Gene: Role in Atherosclerosis. Atherosclerosis 2003, 168, 381–387. [Google Scholar] [CrossRef]
- Robinet, P.; Fradagrada, A.; Monier, M.-N.; Marchetti, M.; Cogny, A.; Moatti, N.; Paul, J.-L.; Vedie, B.; Lamaze, C. Dynamin Is Involved in Endolysosomal Cholesterol Delivery to the Endoplasmic Reticulum: Role in Cholesterol Homeostasis. Traffic 2006, 7, 811–823. [Google Scholar] [CrossRef]
- Iritani, N.; Nagashima, K.; Fukuda, H.; Katsurada, A.; Tanaka, T. Effects of Dietary Proteins on Lipogenic Enzymes in Rat Liver. J. Nutr. 1986, 116, 190–197. [Google Scholar] [CrossRef]
- Iritani, N.; Hosomi, H.; Fukuda, H.; Tada, K.; Ikeda, H. Soybean Protein Suppresses Hepatic Lipogenic Enzyme Gene Expression in Wistar Fatty Rats. J. Nutr. 1996, 126, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, A.; Fukui, K.; Kojima, M.; Kishida, K.; Maeda, N.; Nagaretani, H.; Hibuse, T.; Nishizawa, H.; Kihara, S.; Waki, M.; et al. Divergent Effects of Soy Protein Diet on the Expression of Adipocytokines. Biochem. Biophys. Res. Commun. 2003, 311, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Wood, C.; L’Abbé, M.R.; Gilani, G.S.; Cooke, G.M.; Curran, I.H.; Xiao, C.W. Consumption of Soy Protein Isolate Modulates the Phosphorylation Status of Hepatic ATPase/ATP Synthase β Protein and Increases ATPase Activity in Rats. J. Nutr. 2007, 137, 2029–2035. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.C.; Park, K.; Lopez-Casillas, F.; Kim, K.H. Structural Features of the Acetyl-CoA Carboxylase Gene: Mechanisms for the Generation of mRNAs with 5′ End Heterogeneity. Proc. Natl. Acad. Sci. USA 1989, 86, 4042–4046. [Google Scholar] [CrossRef] [Green Version]
- Aoki, H.; Kimura, K.; Igarashi, K.; Takenaka, A. Soy Protein Suppresses Gene Expression of Acetyl-CoA Carboxylase Alpha from Promoter PI in Rat Liver. Biosci. Biotechnol. Biochem. 2006, 70, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Standeven, A.; Beard, R.; Johnson, A.; Boehm, M.; Escobar, M.; Heyman, R.; Chandraratna, R. Retinoid-Induced Hypertriglyceridemia in Rats Is Mediated by Retinoic Acid Receptors. Fundam. Appl. Toxicol. 1996, 33, 264–271. [Google Scholar] [CrossRef]
- Xiao, C.W. Health Effects of Soy Protein and Isoflavones in Humans. J. Nutr. 2008, 138, 1244S–1249S. [Google Scholar] [CrossRef] [Green Version]
- Lovati, M.; Manzoni, C.; Agostinelli, P.; Ciapellano, S.; Sirtori, C.R. Studies on the Mechanism of the Cholesterol Lowering Activity of Soy Proteins. Nutr. Metab. Cardiovasc. Dis. 1991, 1, 18–24. [Google Scholar]
- Baum, J.A.; Teng, H.; Erdman, J.W., Jr.; Weigel, R.M.; Klein, B.P.; Persky, V.W.; Freels, S.; Surya, P.; Bakhit, R.M.; Ramos, E.; et al. Long-Term Intake of Soy Protein Improves Blood Lipid Profiles and Increases Mononuclear Cell Low-Density-Lipoprotein Receptor Messenger RNA in Hypercholesterolemic, Postmenopausal Women. Am. J. Clin. Nutr. 1998, 68, 545–551. [Google Scholar] [CrossRef]
- Lovati, M.R.; Manzoni, C.; Canavesi, A.; Sirtori, M.; Vaccarino, V.; Marchi, M.; Gaddi, A.; Sirtori, C.R. Soybean Protein Diet Increases Low Density Lipoprotein Receptor Activity in Mononuclear Cells from Hypercholesterolemic Patients. J. Clin. Investig. 1987, 80, 125. [Google Scholar] [CrossRef] [Green Version]
- Ip, E.; Farrell, G.C.; Robertson, G.; Hall, P.; Kirsch, R.; Leclercq, I. Central Role of PPARα-Dependent Hepatic Lipid Turnover in Dietary Steatohepatitis in Mice. Hepatology 2003, 38, 123–132. [Google Scholar] [CrossRef]
- Morifuji, M.; Sanbongi, C.; Sugiura, K. Dietary Soya Protein Intake and Exercise Training Have an Additive Effect on Skeletal Muscle Fatty Acid Oxidation Enzyme Activities and mRNA Levels in Rats. Br. J. Nutr. 2006, 96, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Conti, R.; Mannucci, E.; Pessotto, P.; Tassoni, E.; Carminati, P.; Giannessi, F.; Arduini, A. Selective Reversible Inhibition of Liver Carnitine Palmitoyl-Transferase 1 by Teglicar Reduces Gluconeogenesis and Improves Glucose Homeostasis. Diabetes 2011, 60, 644. [Google Scholar] [CrossRef] [Green Version]
- Lionetti, V.; Linke, A.; Chandler, M.P.; Young, M.E.; Penn, M.S.; Gupte, S.; d’Agostino, C.; Hintze, T.H.; Stanley, W.C.; Recchia, F.A. Carnitine Palmitoyl Transferase-I Inhibition Prevents Ventricular Remodeling and Delays Decompensation in Pacing-Induced Heart Failure. Cardiovasc. Res. 2005, 66, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Fraulob, J.C.; Souza-Mello, V.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Beneficial Effects of Rosuvastatin on Insulin Resistance, Adiposity, Inflammatory Markers and Non-Alcoholic Fatty Liver Disease in Mice Fed on a High-Fat Diet. Clin. Sci. 2012, 123, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenenbaum, A.; Motro, M.; Fisman, E.Z. Dual and Pan-Peroxisome Proliferator-Activated Receptors (PPAR) Co-Agonism: The Bezafibrate Lessons. Cardiovasc. Diabetol. 2005, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa-Da-Silva, S.; Souza-Mello, V.; Magliano, D.C.; Marinho, T.D.S.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. Singular Effects of PPAR Agonists on Nonalcoholic Fatty Liver Disease of Diet-Induced Obese Mice. Life Sci. 2015, 127, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Pellieux, C.; Montessuit, C.; Papageorgiou, I.; Lerch, R. Angiotensin II Downregulates the Fatty Acid Oxidation Pathway in Adult Rat Cardiomyocytes via Release of Tumour Necrosis Factor-α. Cardiovasc. Res. 2009, 82, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Kimura, R.; Takahashi, N.; Murota, K.; Yamada, Y.; Niiya, S.; Kanzaki, N.; Murakami, Y.; Moriyama, T.; Goto, T.; Kawada, T. Activation of Peroxisome Proliferator-Activated Receptor-α (PPARα) Suppresses Postprandial Lipidemia through Fatty Acid Oxidation in Enterocytes. Biochem. Biophys. Res. Commun. 2011, 410, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Osorio, J.; Stanley, W.; Linke, A.; Castellari, M.; Quy, D.; Panchal, A.; Hintze, T.; Lopaschuk, G.; Recchia, F. Impaired Myocardial Fatty Acid Oxidation and Reduced Protein Expression of Retinoid X Receptor-α in Pacing-Induced Heart Failure. Circulation 2002, 106, 606–612. [Google Scholar] [CrossRef] [Green Version]
- Bishop-Bailey, D. Peroxisome Proliferator-Activated Receptors in the Cardiovascular System. Br. J. Pharmacol. 2000, 129, 823–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, K.; Fujii, H.; Takahashi, T.; Kodama, M.; Aizawa, Y.; Ohta, Y.; Ono, T.; Hasegawa, G.; Naito, M.; Nakajima, T.; et al. Constitutive Regulation of Cardiac Fatty Acid Metabolism through Peroxisome Proliferator-Activated Receptor α Associated with Age-Dependent Cardiac Toxicity. J. Biol. Chem. 2000, 275, 22293–22299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, H.; Dreyer, C.; Medin, J.; Mahfoudi, A.; Ozato, K.; Wahli, W. Fatty Acids and Retinoids Control Lipid Metabolism through Activation of Peroxisome Proliferator-Activated Receptor-Retinoid X Receptor Heterodimers. Proc. Natl. Acad. Sci. USA 1993, 90, 2160–2164. [Google Scholar] [CrossRef] [Green Version]
- Tugwood, J.D.; Issemann, I.; Anderson, R.G.; Bundell, K.R.; McPheat, W.L.; Green, S. The Mouse Peroxisome Proliferator Activated Receptor Recognizes a Response Element in the 5′ Flanking Sequence of the Rat Acyl CoA Oxidase Gene. EMBO J. 1992, 11, 433–439. [Google Scholar] [CrossRef]
- Dreyer, C.; Krey, G.; Keller, H.; Givel, F.; Helftenbein, G.; Wahli, W. Control of the Peroxisomal β-Oxidation Pathway by a Novel Family of Nuclear Hormone Receptors. Cell 1992, 68, 879–887. [Google Scholar] [CrossRef]
- Huss, J.M.; Levy, F.H.; Kelly, D.P. Hypoxia Inhibits the Peroxisome Proliferator-Activated Receptor α/Retinoid X Receptor Gene Regulatory Pathway in Cardiac Myocytes: A Mechanism for O2-Dependent Modulation of Mitochondrial Fatty Acid Oxidation. J. Biol. Chem. 2001, 276, 27605–27612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sack, M.; Rader, T.; Park, S.; Bastin, J.; McCune, S.; Kelly, D. Fatty Acid Oxidation Enzyme Gene Expression Is Downregulated in the Failing Heart. Circulation 1996, 94, 2837–2842. [Google Scholar] [CrossRef]
- Razeghi, P.; Young, M.E.; Alcorn, J.L.; Moravec, C.; Frazier, O.; Taegtmeyer, H. Metabolic Gene Expression in Fetal and Failing Human Heart. Circulation 2001, 104, 2923–2931. [Google Scholar] [CrossRef] [Green Version]
- Schupp, M.; Kintscher, U.; Fielitz, J.; Thomas, J.; Pregla, R.; Hetzer, R.; Unger, T.; Regitz-Zagrosek, V. Cardiac PPARα Expression in Patients with Dilated Cardiomyopathy. Eur. J. Heart Fail. 2006, 8, 290–294. [Google Scholar] [CrossRef]
- Milanski, M.; Souza, K.L.A.; Reis, S.R.L.; Feres, N.H.; de Souza, L.M.I.; Arantes, V.C.; Carneiro, E.M.; Boschero, A.C.; Reis, M.A.B.; Latorraca, M.Q. Soybean Diet Modulates Acetyl-Coenzyme A Carboxylase Expression in Livers of Rats Recovering from Early-Life Malnutrition. Nutrition 2009, 25, 774–781. [Google Scholar] [CrossRef]
- Katsurada, A.; Iritani, N.; Fukuda, H.; Matsumura, Y.; Nishimoto, N.; Noguchi, T.; Tanaka, T. Effects of Nutrients and Hormones on Transcriptional and Post-Transcriptional Regulation of Fatty Acid Synthase in Rat Liver. Eur. J. Biochem. 1990, 190, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Seo, C.-S.; Hwang, I.H.; Lee, M.W.; Song, K.H. Anti-Obesity Activities of Chikusetsusaponin IVa and Dolichos lablab L. Seeds. Nutrients 2018, 10, 1221. [Google Scholar] [CrossRef] [Green Version]
- Barak, Y.; Nelson, M.C.; Ong, E.S.; Jones, Y.Z.; Ruiz-Lozano, P.; Chien, K.R.; Koder, A.; Evans, R.M. PPARγ Is Required for Placental, Cardiac, and Adipose Tissue Development. Mol. Cell 1999, 4, 585–595. [Google Scholar] [CrossRef]
- Schoonjans, K.; Peinado-Onsurbe, J.; Lefebvre, A.M.; Heyman, R.A.; Briggs, M.; Deeb, S.; Staels, B.; Auwerx, J. PPARalpha and PPARgamma Activators Direct a Distinct Tissue-Specific Transcriptional Response via a PPRE in the Lipoprotein Lipase Gene. EMBO J. 1996, 15, 5336–5348. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Association. Food labeling: Health claims; soybean protein and coronary heart disease. In Federal. Register: Rules and Regulations; Food and Drug Administration: Silver Spring, MD, USA, 1999; Volume 64, pp. 57700–57733. [Google Scholar]
- Harland, J.I.; Haffner, T.A. Systematic Review, Meta-Analysis and Regression of Randomised Controlled Trials Reporting an Association Between an Intake of Circa 25g Soya Protein per Day and Blood Cholesterol. Atherosclerosis 2008, 200, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Benkhedda, K.; Boudrault, C.; Sinclair, S.; Marles, R.; Xiao, C.; Underhill, L. Food Risk Analysis Communication. Issued by Health Canada’s Food Directorate. Health Canada’s Proposal to Accept a Health Claim about Soy Products and Cholesterol Lowering. Int. Food Risk Anal. J. 2014, 4, 1–12. [Google Scholar]
- Nagarajan, S.; Burris, R.L.; Stewart, B.W.; Wilkerson, J.E.; Badger, T.M. Dietary Soy Protein Isolate Ameliorates Atherosclerotic Lesions in Apolipoprotein E-Deficient Mice Potentially by Inhibiting Monocyte Chemoattractant Protein-1 Expression. J. Nutr. 2008, 138, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Kwon, D.; Oh, S.; Lee, J.; Yang, H.; Lee, S.; Lee, J.; Lee, Y.; Sohn, H. Amino Acid Substitution of Hypocholesterolemic Peptide Originated from Glycinin Hydrolyzate. Food Sci. Biotechnol. 2002, 11, 55–61. [Google Scholar]
- Farrokhi, N.; Whitelegge, J.P.; Brusslan, J.A. Plant Peptides and Peptidomics. Plant Biotechnol. J. 2008, 6, 105–134. [Google Scholar] [CrossRef]
- Nasri, R.; Nasri, M. Marine-Derived Bioactive Peptides as New Anticoagulant Agents: A Review. Curr. Protein Pept. Sci. 2013, 14, 199–204. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Pinto, D.; Gobbetti, M. Selected Lactic Acid Bacteria Synthesize Antioxidant Peptides during Sourdough Fermentation of Cereal Flours. Appl. Environ. Microbiol. 2012, 78, 1087. [Google Scholar] [CrossRef] [Green Version]
- Korhonen, H.; Pihlanto, A. Food-Derived Bioactive Peptides—Opportunities for Designing Future Foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [Google Scholar] [CrossRef] [Green Version]
- Tsai, J.S.; Lin, Y.S.; Pan, B.S.; Chen, T.J. Antihypertensive Peptides and γ-Aminobutyric Acid from Prozyme 6 Facilitated Lactic Acid Bacteria Fermentation of Soymilk. Process Biochem. 2006, 41, 1282–1288. [Google Scholar] [CrossRef]
- Tsai, J.-S.; Chen, T.-J.; Pan, B.S.; Gong, S.-D.; Chung, M.-Y. Antihypertensive Effect of Bioactive Peptides Produced by Protease-Facilitated Lactic Acid Fermentation of Milk. Food Chem. 2008, 106, 552–558. [Google Scholar] [CrossRef]
- Zambrowicz, A.; Timmer, M.; Polanowski, A.; Lubec, G.; Trziszka, T. Manufacturing of Peptides Exhibiting Biological Activity. Amino Acids 2013, 44, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Agyei, D. Bioactive Proteins and Peptides from Soybeans. Recent. Pat. Food Nutr. Agric. 2015, 7, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ledesma, B.; Hsieh, C.-C.; De Lumen, B.O. Chemopreventive Properties of Peptide Lunasin: A Review. Protein Pept. Lett. 2013, 20, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Shimakage, A.; Shinbo, M.; Yamada, S. ACE Inhibitory Substances Derived from Soy Foods. J. Biol. Macromol. 2012, 12, 72–80. [Google Scholar] [CrossRef] [Green Version]
- S Vallabha, V.; Tiku, P.K. Antihypertensive Peptides Derived from Soy Protein by Fermentation. Int. J. Pept. Res. Ther. 2014, 20, 161–168. [Google Scholar] [CrossRef]
- Johnston, J.; Franz, V. Renin-Angiotensin System: A Dual Tissue and Hormonal System for Cardiovascular Control. J. Hypertens. 1992, 10, 13–26. [Google Scholar] [CrossRef]
- Nguyen Dinh Cat, A.; Touyz, R.M. A New Look at the Renin–Angiotensin System—Focusing on the Vascular System. Peptides 2011, 32, 2141–2150. [Google Scholar] [CrossRef]
- Jao, C.L.; Huang, S.L.; Hsu, K.C. Angiotensin I-Converting Enzyme Inhibitory Peptides: Inhibition Mode, Bioavailability, and Antihypertensive Effects. BioMedicine 2012, 2, 130–136. [Google Scholar] [CrossRef]
- Sibony, M.; Gasc, J.M.; Soubrier, F.; Alhenc-Gelas, F.; Corvol, P. Gene Expression and Tissue Localization of the Two Isoforms of Angiotensin I Converting Enzyme. Hypertension 1993, 21, 827–835. [Google Scholar] [CrossRef] [Green Version]
- Natesh, R.; Schwager, S.L.U.; Sturrock, E.D.; Acharya, K.R. Crystal Structure of the Human Angiotensin-Converting Enzyme–Lisinopril Complex. Nature 2003, 421, 551–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donkor, O.N.; Henriksson, A.; Singh, T.K.; Vasiljevic, T.; Shah, N.P. ACE-Inhibitory Activity of Probiotic Yoghurt. Int. Dairy J. 2007, 17, 1321–1331. [Google Scholar] [CrossRef]
- Mallikarjun Gouda, K.G.; Gowda, L.R.; Rao, A.G.A.; Prakash, V. Angiotensin I-Converting Enzyme Inhibitory Peptide Derived from Glycinin, the 11S Globulin of Soybean (Glycine max). J. Agric. Food Chem. 2006, 54, 4568–4573. [Google Scholar] [CrossRef]
- Prak, K.; Maruyama, Y.; Maruyama, N.; Utsumi, S. Design of Genetically Modified Soybean Proglycinin A1aB1b with Multiple Copies of Bioactive Peptide Sequences. Peptides 2006, 27, 1179–1186. [Google Scholar] [CrossRef]
- Nagaoka, S. Structure-Function Properties of Hypolipidemic Peptides. J. Food Biochem. 2019, 43, e12539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, M.; Uy, L.; Manuel, M.; Recuenco, M.; Torio, M. Hypocholesterolemic Activity of Mungbean 8Sα Globulin Engineered with Lactostatin. PJCS 2020, 45, 13–26. [Google Scholar]
- Odani, S.; Koide, T.; Ono, T. Amino Acid Sequence of a Soybean (Glycine max) Seed Polypeptide Having a Poly(L-Aspartic Acid) Structure. J. Biol. Chem. 1987, 262, 10502–10505. [Google Scholar] [CrossRef]
- Galvez, A.F.; de Lumen, B.O. A Soybean cDNA Encoding a Chromatin-Binding Peptide Inhibits Mitosis of Mammalian Cells. Nat. Biotechnol. 1999, 17, 495–500. [Google Scholar] [CrossRef] [PubMed]
- De Lumen, B.O. Lunasin: A Cancer-Preventive Soy Peptide. Nutr. Rev. 2005, 63, 16–21. [Google Scholar] [CrossRef]
- Lule, V.K.; Garg, S.; Pophaly, S.D.; Hitesh; Tomar, S.K. Potential Health Benefits of Lunasin: A Multifaceted Soy-Derived Bioactive Peptide. J. Food Sci. 2015, 80, R485–R494. [Google Scholar] [CrossRef]
- Seber, L.E.; Barnett, B.W.; McConnell, E.J.; Hume, S.D.; Cai, J.; Boles, K.; Davis, K.R. Scalable Purification and Characterization of the Anticancer Lunasin Peptide from Soybean. PLoS ONE 2012, 7, e35409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayaprolu, S.J.; Hettiarachchy, N.S.; Chen, P.; Kannan, A.; Mauromostakos, A. Peptides Derived from High Oleic Acid Soybean Meals Inhibit Colon, Liver and Lung Cancer Cell Growth. Food Res. Int. 2013, 50, 282–288. [Google Scholar] [CrossRef]
- Park, J.H.; Jeong, H.J.; de Lumen, B.O. Contents and Bioactivities of Lunasin, Bowman−Birk Inhibitor, and Isoflavones in Soybean Seed. J. Agric. Food Chem. 2005, 53, 7686–7690. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Jeong, J.B.; Kim, D.S.; Park, J.H.; Lee, J.B.; Kweon, D.-H.; Chung, G.Y.; Seo, E.W.; de Lumen, B.O. The Cancer Preventive Peptide Lunasin from Wheat Inhibits Core Histone Acetylation. Cancer Lett. 2007, 255, 42–48. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, J.; Jeong, J.; Park, J.; Cheong, Y.; de Lumen, B. The Preventive Seed Peptide Lunasin from Rye Is Bioavailable and Bioactive. Nutr. Cancer 2009, 61, 680–686. [Google Scholar] [CrossRef]
- Jeong, H.J.; Jeong, J.B.; Hsieh, C.C.; Hernández-Ledesma, B.; de Lumen, B.O. Lunasin Is Prevalent in Barley and Is Bioavailable and Bioactive in in Vivo and in Vitro Studies. Nutr. Cancer 2010, 62, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- García-Nebot, M.; Recio, I.; Hernández-Ledesma, B. Antioxidant Activity and Protective Effects of Peptide Lunasin against Oxidative Stress in Intestinal Caco-2 Cells. Food Chem. Toxicol. 2014, 65, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.B.; De Lumen, B.O.; Jeong, H.J. Lunasin Peptide Purified from Solanum nigrum L. Protects DNA from Oxidative Damage by Suppressing the Generation of Hydroxyl Radical via Blocking Fenton Reaction. Cancer Lett. 2010, 293, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; Hsieh, C.-C.; de Lumen, B.O. Antioxidant and Anti-Inflammatory Properties of Cancer Preventive Peptide Lunasin in RAW 264.7 Macrophages. Biochem. Biophys. Res. Commun. 2009, 390, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Dia, V.P.; Wang, W.; Oh, V.L.; de Lumen, B.O.; de Mejia, E.G. Isolation, Purification and Characterisation of Lunasin from Defatted Soybean Flour and in Vitro Evaluation of Its Anti-Inflammatory Activity. Food Chem. 2009, 114, 108–115. [Google Scholar] [CrossRef]
- Aguzzi, M.S.; Fortugno, P.; Giampietri, C.; Ragone, G.; Capogrossi, M.C.; Facchiano, A. Intracellular Targets of RGDS Peptide in Melanoma Cells. Mol. Cancer 2010, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Gueguen, J.; Cerletti, P. Proteins of some legume seeds: Soybean bean, pea, faba bean and lupin. In New and Developing Sources of Food Proteins; Chapman and Hall: London, UK, 1994; pp. 145–193. ISBN 978-1-4615-2652-0. [Google Scholar]
- Katagiri, Y.; Ibrahim, R.; Tahara, S. HPLC Analysis of White Lupin Isoflavonoids. Biosci. Biotechnol. Biochem. 2000, 64, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Sujak, A.; Kotlarz, A.; Strobel, W. Compositional and Nutritional Evaluation of Several Lupin Seeds. Food Chem. 2006, 98, 711–719. [Google Scholar] [CrossRef]
- Bettzieche, A.; Brandsch, C.; Weiße, K.; Hirche, F.; Eder, K.; Stangl, G. Lupin Protein Influences the Expression of Hepatic Genes Involved in Fatty Acid Synthesis and Triacylglycerol Hydrolysis of Adult Rats. Br. J. Nutr. 2008, 99, 952–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boschin, G.; D’Agostina, A.; Annicchiarico, P.; Arnoldi, A. Effect of Genotype and Environment on Fatty Acid Composition of Lupinus Albus L. Seed. Food Chem. 2008, 108, 600–606. [Google Scholar] [CrossRef]
- Boschin, G.; Arnoldi, A. Legumes Are Valuable Sources of Tocopherols. Food Chem. 2011, 127, 1199–1203. [Google Scholar] [CrossRef]
- Brandsch, C.; Kappis, D.; Weiße, K.; Stangl, G.I. Effects of Untreated and Thermally Treated Lupin Protein on Plasma and Liver Lipids of Rats Fed a Hypercholesterolemic High Fat or High Carbohydrate Diet. Plant Foods Hum. Nutr. 2010, 65, 410–416. [Google Scholar] [CrossRef]
- Spielmann, J.; Shukla, A.; Brandsch, C.; Hirche, F.; Stangl, G.I.; Eder, K. Dietary Lupin Protein Lowers Triglyceride Concentrations in Liver and Plasma in Rats by Reducing Hepatic Gene Expression of Sterol Regulatory Element-Binding Protein-1c. Ann. Nutr. Metab. 2007, 51, 387–392. [Google Scholar] [CrossRef]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef]
- Naruszewicz, M.; Nowicka, G.; Klosiewicz-Latoszek, L.; Arnoldi, A.; Sirtori, C. Abstract 4055: Effect of Lupin Protein (Lupinus albus) on Cardiovascular Risk Factors in Smokers with Mild Hypercholesterolemia. Circulation 2006, 114, II_874. [Google Scholar] [CrossRef]
- Bettzieche, A.; Brandsch, C.; Schmidt, M.; Weiße, K.; Eder, K.; Stangl, G. Differing Effect of Protein Isolates from Different Cultivars of Blue Lupin on Plasma Lipoproteins of Hypercholesterolemic Rats. Biosci. Biotechnol. Biochem. 2008, 72, 3114–3121. [Google Scholar] [CrossRef] [Green Version]
- Sirtori, C.; Galli, C.; Anderson, J.W.; Arnoldi, A. Nutritional and Nutraceutical Approaches to Dyslipidemia and Atherosclerosis Prevention: Focus on Dietary Proteins. Atherosclerosis 2009, 203, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Zanoni, C.; Scigliuolo, G.M.; D’Amato, A.; Arnoldi, A. Lupin Peptides Lower Low-Density Lipoprotein (LDL) Cholesterol Through an Up-Regulation of the LDL Receptor/Sterol Regulatory Element Binding Protein 2 (SREBP2) Pathway at HepG2 Cell Line. J. Agric. Food Chem. 2014, 62, 7151–7159. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Triolo, M.; Bosisio, R.; Bondioli, A.; Calabresi, L.; De Vergori, V.; Gomaraschi, M.; Mombelli, G.; Pazzucconi, F.; Zacherl, C.; et al. Hypocholesterolaemic Effects of Lupin Protein and Pea Protein/Fibre Combinations in Moderately Hypercholesterolaemic Individuals. Br. J. Nutr. 2012, 107, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Blagrove, R.; Gillespie, J. Isolation, Purification and Characterization of the Seed Globulins of Lupinus albus. Aust. J. Plant Physiol. 1981, 2, 13–27. [Google Scholar]
- Duranti, M.; Restani, P.; Poniatowska, M.; Cerletti, P. The Seed Globulins of Lupinus albus. Phytochemistry 1981, 20, 2071–2075. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Ferruzza, S.; Ranaldi, G.; Sambuy, Y.; Arnoldi, A. Hypocholesterolaemic Activity of Lupin Peptides: Investigation on the Crosstalk between Human Enterocytes and Hepatocytes Using a Co-Culture System Including Caco-2 and HepG2 Cells. Nutrients 2016, 8, 437. [Google Scholar] [CrossRef] [Green Version]
- Lammi, C.; Zanoni, C.; Aiello, G.; Arnoldi, A.; Grazioso, G. Lupin Peptides Modulate the Protein-Protein Interaction of PCSK9 with the Low Density Lipoprotein Receptor in HepG2 Cells. Sci. Rep. 2016, 6, 29931. [Google Scholar] [CrossRef]
- Bähr, M.; Fechner, A.; Kiehntopf, M.; Jahreis, G. Consuming a Mixed Diet Enriched with Lupin Protein Beneficially Affects Plasma Lipids in Hypercholesterolemic Subjects: A Randomized Controlled Trial. Clin. Nutr. 2015, 34, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the Health Benefits of Peas (Pisum sativum L.). Br. J. Nutr. 2012, 108, S3–S10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimm, E.B.; Ascherio, A.; Giovannucci, E.; Spiegelman, D.; Stampfer, M.J.; Willett, W.C. Vegetable, Fruit, and Cereal Fiber Intake and Risk of Coronary Heart Disease among Men. JAMA 1996, 275, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Tzitzikas, E.N.; Vincken, J.-P.; de Groot, J.; Gruppen, H.; Visser, R.G.F. Genetic Variation in Pea Seed Globulin Composition. J. Agric. Food Chem. 2006, 54, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Hood-Niefer, S.D.; Warkentin, T.D.; Chibbar, R.N.; Vandenberg, A.; Tyler, R.T. Effect of Genotype and Environment on the Concentrations of Starch and Protein in, and the Physicochemical Properties of Starch from, Field Pea and Fababean. J. Sci. Food Agric. 2012, 92, 141–150. [Google Scholar] [CrossRef]
- Teunissen-Beekman, K.F.M.; Dopheide, J.; Geleijnse, J.M.; Bakker, S.J.L.; Brink, E.J.; de Leeuw, P.W.; Serroyen, J.; van Baak, M.A. Differential Effects of Proteins and Carbohydrates on Postprandial Blood Pressure-Related Responses. Br. J. Nutr. 2014, 112, 600–608. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Bhullar, K.S.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Egg White-Derived Tripeptide IRW (Ile-Arg-Trp) Is an Activator of Angiotensin Converting Enzyme 2. J. Agric. Food Chem. 2018, 66, 11330–11336. [Google Scholar] [CrossRef]
- Majumder, K.; Liang, G.; Chen, Y.; Guan, L.; Davidge, S.T.; Wu, J. Egg Ovotransferrin-Derived ACE Inhibitory Peptide IRW Increases ACE2 but Decreases Proinflammatory Genes Expression in Mesenteric Artery of Spontaneously Hypertensive Rats. Mol. Nutr. Food Res. 2015, 59, 1735–1744. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Modulatory Effects of Egg White Ovotransferrin-Derived Tripeptide IRW (Ile-Arg-Trp) on Vascular Smooth Muscle Cells against Angiotensin II Stimulation. J. Agric. Food Chem. 2016, 64, 7342–7347. [Google Scholar] [CrossRef]
- Khalid, I.; Elharadallou, S. Functional Properties of Cowpea (Vigna ungiculata L. Walp), and Lupin (Lupinus termis) Flour and Protein Isolates. J. Nutr. Food Sci. 2013, 3, 1–6. [Google Scholar]
- Kirse, A.; Karklina, D. Integrated Evaluation of Cowpea (Vigna unguiculata (L.) Walp.) and Maple Pea (Pisum sativum var. Arvense L.) Spreads. Agron. Res. 2015, 13, 956–968. [Google Scholar]
- Sebetha, E.; Modi, A.; Owoeye, L. Cowpea Crude Protein as Affected by Cropping System, Site and Nitrogen Fertilization. J. Agric. Sci. 2014, 7, 224–234. [Google Scholar] [CrossRef] [Green Version]
- Trehan, I.; Benzoni, N.S.; Wang, A.Z.; Bollinger, L.B.; Ngoma, T.N.; Chimimba, U.K.; Stephenson, K.B.; Agapova, S.E.; Maleta, K.M.; Manary, M.J. Common Beans and Cowpeas as Complementary Foods to Reduce Environmental Enteric Dysfunction and Stunting in Malawian Children: Study Protocol for Two Randomized Controlled Trials. Trials 2015, 16, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, A.; Goufo, P.; Barros, A.; Domínguez-Perles, R.; Trindade, H.; Rosa, E.A.S.; Ferreira, L.; Rodrigues, M. Cowpea (Vigna unguiculata L. Walp), a Renewed Multipurpose Crop for a More Sustainable Agri-Food System: Nutritional Advantages and Constraints. J. Sci. Food Agric. 2016, 96, 2941–2951. [Google Scholar] [CrossRef]
- Petchiammal, C.; Hopper, W. Antioxidant Activity of Proteins from Fifteen Varieties of Legume Seeds Commonly Consumed in India. Int. J. Pharm. 2014, 6, 476–479. [Google Scholar]
- Kanetro, B. Hypocholesterolemic Properties of Protein Isolate from Cowpeas (Vigna unguiculata) Sprout in Normal and Diabetic Rats. Procedia Food Sci. 2015, 3, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Frota, K.; Matias, A.; Areas, J. Influence of Food Components on Lipid Metabolism: Scenarios and Perspective on the Control and Prevention of Dyslipidemias. Food Sci. Technol. 2010, 30, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Frota, K.; dos Santos, F.; Ribeiro, V.; Areas, J. Cowpea Protein Reduces LDL-Cholesterol and Apolipoprotein B Concentrations, but Does Not Improve Biomarkers of Inflammation or Endothelial Dysfunction in Adults with Moderate Hypercholesterolemia. Nutr. Hosp. 2015, 31, 1611–1619. [Google Scholar]
- Marques, M.R.; Cerda, A.; Fontanari, G.G.; Pimenta, D.C.; Soares-Freitas, R.M.; Hirata, M.H.; Hirata, R.D.C.; Arêas, J.A.G. Transport of Cowpea Bean Derived Peptides and Their Modulator Effects on mRNA Expression of Cholesterol-Related Genes in Caco-2 and HepG2 Cells. Food Res. Int. 2018, 107, 165–171. [Google Scholar] [CrossRef]
- Marques, M.R.; Soares Freitas, R.A.M.; Corrêa Carlos, A.C.; Siguemoto, É.S.; Fontanari, G.G.; Arêas, J.A.G. Peptides from Cowpea Present Antioxidant Activity, Inhibit Cholesterol Synthesis and Its Solubilisation into Micelles. Food Chem. 2015, 168, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Real-Hernandez, L.M.; Gonzalez de Mejia, E. Bean Peptides Have Higher In Silico Binding Affinities than Ezetimibe for the N-Terminal Domain of Cholesterol Receptor Niemann-Pick C1 Like-1. Peptides 2017, 90, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; Singh, R.; Malhotra, S.; Boora, K.S.; Singal, H.R. Characterization of Seed Storage Proteins in High Protein Genotypes of Cowpea [Vigna unguiculata (L.) Walp]. Physiol. Mol. Biol. Plants 2010, 16, 53–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.J.; Kim, T.W.; Baik, B.-K. Relationship between Proportion and Composition of Albumins, and In Vitro Protein Digestibility of Raw and Cooked Pea Seeds (Pisum sativum L.). J. Sci. Food Agric. 2010, 90, 1719–1725. [Google Scholar] [CrossRef]
- Tchiagam, J.; Bell, J.; Antoine, N.; Njintang, N.; Youmbi, E. Genetic Analysis of Seed Proteins Contents in Cowpea (Vigna unguiculata L Walp). Afr. J. Biotechnol. 2011, 10, 3077–3086. [Google Scholar]
- Hachibamba, T.; Dykes, L.; Awika, J.; Minnaar, A.; Duodu, K.G. Effect of Simulated Gastrointestinal Digestion on Phenolic Composition and Antioxidant Capacity of Cooked Cowpea (Vigna unguiculata) Varieties. Int. J. Food Sci. Technol. 2013, 48, 2638–2649. [Google Scholar] [CrossRef]
- Watanabe, H.; Inaba, Y.; Kimura, K.; Asahara, S.; Kido, Y.; Matsumoto, M.; Motoyama, T.; Tachibana, N.; Kaneko, S.; Kohno, M.; et al. Dietary Mung Bean Protein Reduces Hepatic Steatosis, Fibrosis, and Inflammation in Male Mice with Diet-Induced, Nonalcoholic Fatty Liver Disease. J. Nutr. 2016, 147, 52–60. [Google Scholar] [CrossRef]
- Li, G.; Le, G.; Liu, H.; Shi, Y. Mung-Bean Protein Hydrolysates Obtained with Alcalase Exhibit Angiotensin I-Converting Enzyme Inhibitory Activity. Food Sci. Technol. Int. 2005, 11, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Wongekalak, L.; Sakulsom, P.; Jirasripongpun, K.; Hongsprabhas, P. Potential Use of Antioxidative Mungbean Protein Hydrolysate as an Anticancer Asiatic Acid Carrier. Food Res. Int. 2011, 44, 812–817. [Google Scholar] [CrossRef]
- Xie, J.; Du, M.; Shen, M.; Wu, T.; Lin, L. Physico-Chemical Properties, Antioxidant Activities and Angiotensin-I Converting Enzyme Inhibitory of Protein Hydrolysates from Mung Bean (Vigna radiata). Food Chem. 2019, 270, 243–250. [Google Scholar] [CrossRef]
- Li, G.; Wan, J.; Le, G.; Shi, Y. Novel Angiotensin I-Converting Enzyme Inhibitory Peptides Isolated from Alcalase Hydrolysate of Mung Bean Protein. J. Pept. Sci. 2006, 12, 509–514. [Google Scholar] [CrossRef]
- Clemente, A.; Vioque, J.; Sánchez-Vioque, R.; Pedroche, J.; Bautista, J.; Millán, F. Protein Quality of Chickpea (Cicer arietinum L.) Protein Hydrolysates. Food Chem. 1999, 67, 269–274. [Google Scholar] [CrossRef]
- Pittaway, J.K.; Robertson, I.K.; Ball, M.J. Chickpeas May Influence Fatty Acid and Fiber Intake in an Ad Libitum Diet, Leading to Small Improvements in Serum Lipid Profile and Glycemic Control. J. Am. Diet. Assoc. 2008, 108, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Loarca-Piña, G.; Oomah, B.D. Minor Components of Pulses and Their Potential Impact on Human Health. Food Res. Int. 2010, 43, 461–482. [Google Scholar] [CrossRef]
- Madani, S.; Prost, J.; Belleville, J. Dietary Protein Level and Origin (Casein and Highly Purified Soybean Protein) Affect Hepatic Storage, Plasma Lipid Transport, and Antioxidative Defense Status in the Rat. Nutrition 2000, 16, 368–375. [Google Scholar] [CrossRef]
- Chau, C.; Cheung, P.; Wong, Y. Hypocholesterolemic Effects of Protein Concentrates from Three Chinese Indigenous Legume Seeds. J. Agric. Food Chem. 1998, 46, 3698–3701. [Google Scholar] [CrossRef]
- Duranti, M. Grain Legume Proteins and Nutraceutical Properties. Fitoterapia 2006, 77, 67–82. [Google Scholar] [CrossRef]
- Fernández-Quintela, A.; Macarulla, M.T.; del Barrio, A.S.; Martínez, J.A. Composition and Functional Properties of Protein Isolates Obtained from Commercial Legumes Grown in Northern Spain. Plant Foods Hum. Nutr. 1997, 51, 331–341. [Google Scholar] [CrossRef]
- Fernández-Quintela, A.; Barrio, A.S.D.; Macarulla, M.T.; Martínez, J.A. Nutritional Evaluation and Metabolic Effects in Rats of Protein Isolates Obtained from Seeds of Three Legume Species. J. Sci. Food Agric. 1998, 78, 251–260. [Google Scholar] [CrossRef]
- Li, Y.W.; Li, B.; He, J.; Qian, P. Quantitative Structure–Activity Relationship Study of Antioxidative Peptide by Using Different Sets of Amino Acids Descriptors. J. Mol. Struct. 2011, 998, 53–61. [Google Scholar] [CrossRef]
- Roberts, P.R.; Burney, J.D.; Black, K.W.; Zaloga, G.P. Effect of Chain Length on Absorption of Biologically Active Peptides from the Gastrointestinal Tract. Digestion 1999, 60, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xie, N.; Li, B. Influence of Peptide Characteristics on Their Stability, Intestinal Transport, and In Vitro Bioavailability: A Review. J. Food Biochem. 2019, 43, e12571. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory Peptides: Quantitative Structure−Activity Relationship Study of Di- and Tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef]
- Jang, J.-H.; Jeong, S.-C.; Kim, J.-H.; Lee, Y.-H.; Ju, Y.-C.; Lee, J.-S. Characterisation of a New Antihypertensive Angiotensin I-Converting Enzyme Inhibitory Peptide from Pleurotus cornucopiae. Food Chem. 2011, 127, 412–418. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory Peptides: Quantitative Structure-Activity Relationship Modeling of Peptides Containing 4-10 Amino Acid Residues. QSAR Comb. Sci. 2006, 25, 873–880. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M. Food-Originating ACE Inhibitors, Including Antihypertensive Peptides, as Preventive Food Components in Blood Pressure Reduction. Compr. Rev. Food Sci. Food Saf. 2014, 13, 114–134. [Google Scholar] [CrossRef]
- Temsamani, J.; Vidal, P. The Use of Cell-Penetrating Peptides for Drug Delivery. Drug. Discov. Today 2004, 9, 1012–1019. [Google Scholar] [CrossRef]
- Guha, S.; Majumder, K. Structural-Features of Food-Derived Bioactive Peptides with Anti-Inflammatory Activity: A Brief Review. J. Food Chem. 2019, 43, e12531. [Google Scholar] [CrossRef]
- Nan, Y.; Park, K.; Park, Y.; Jeon, Y.J.; Kim, Y.; Park, I.; Hahm, K.; Shin, S. Investigating the Effects of Positive Charge and Hydrophobicity on the Cell Selectivity, Mechanism of Action and Anti-Inflammatory Activity of a Trp-Rich Antimicrobial Peptide Indolicidin. FEMS. Microbiol. Lett. 2009, 292, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Kyte, J.; Doolittle, R.F. A Simple Method for Displaying the Hydropathic Character of a Protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [Green Version]
- Stothard, P. The Sequence Manipulation Suite: JavaScript Programs for Analyzing and Formatting Protein and DNA Sequences. BioTechniques 2000, 28, 1102–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.; Yang, J. Analysis and Prediction of Highly Effective Antiviral Peptides Based on Random Forests. PLoS ONE 2013, 8, e70166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yea, C.S.; Ebrahimpour, A.; Hamid, A.A.; Bakar, J.; Muhammad, K.; Saari, N. Winged Bean [Psophorcarpus tetragonolobus (L.) DC] Seeds as an Underutilised Plant Source of Bifunctional Proteolysate and Biopeptides. Food Funct. 2014, 5, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Montojo, J.; Zuberi, K.; Rodriguez, H.; Kazi, F.; Wright, G.; Donaldson, S.L.; Morris, Q.; Bader, G.D. GeneMANIA Cytoscape Plugin: Fast Gene Function Predictions on the Desktop. Bioinformatics 2010, 26, 2927–2928. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulos, K.; Viteri, G.; Sevilla, C.; Jupe, S.; Webber, M.; Orlic-Milacic, M.; Jassal, B.; May, B.; Shamovsky, V.; Duenas, C.; et al. Reactome Enhanced Pathway Visualization. Bioinformatics 2017, 33, 3461–3467. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Kanehisa, M. Toward Understanding the Origin and Evolution of Cellular Organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating Viruses and Cellular Organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef]





| Common Name | Scientific Name | Distribution | Uses |
|---|---|---|---|
| Adzuki bean or red mungbean | Vigna angularis (Willd.) Ohwi and Ohashi | Asia, USA, South America, New Zealand, Africa | For human consumption |
| Chickpea | Cicer arietinum L. | Mediterranean countries, South Asia, Eastern and Southern Africa | For human consumption and as animal feed |
| Common bean | Phaseolus vulgaris L. | India, Brazil, France, Russia, German, UK, Ukraine | For human consumption |
| Cowpea | Vigna unguiculata L. | Mediterranean area, Africa, Asia | For human consumption |
| Faba bean | Vicia faba L. | Central Asia, Mediterranean countries, South America, Near East, Europe | For human consumption and as animal feed |
| Jack bean | Canavalia ensiformis (L.) DC. | India, Far East, North and East Africa | For human consumption |
| Lentil | Lens culinaris Medik. | Turkey, Europe (France, Spain), Asia, Canada, USA | For human consumption |
| Lupin | Lupinus spp. | Europe, America | For animal feed |
| Moth bean | Vigna aconitifolia Jacq. | India, USA, Australia, Thailand, other parts of Asia | For human consumption and animal feed |
| Mungbean | Vigna radiata L. | South Asia, China, India | For human consumption |
| Pea | Pisum sativum L. | Europe, North America | For human consumption and animal feed |
| Soybean | Glycine max L. | USA, Brazil, China, Argentina, Japan | For human consumption and for animal feed |
| Legume (Scientific Name) | Component/ Protein | Cardiovascular Association | Experimental Model (If Applicable) | Reference |
|---|---|---|---|---|
| Soybean (Glycine max L.) | Total crude | decreased TC, VLDL-C and TAGs | rat | [100,101,102,103,104] |
| ACE inhibition | rat | [105] | ||
| decreased TAGs and LDL | mouse | [106] | ||
| decreased TC, LDL, TAGs | human | [107,108,109,110,111] | ||
| decreased serum TC | human, rat | [112,113,114,115,116,117,118,119] | ||
| decreased TC, LDL-C, TAGs; increased HDL-C | human | [96,120] | ||
| decreased LDL | human | [111] | ||
| Enzyme digested soy proteins | inhibited lipid peroxidation | [121,122,123,124,125] | ||
| Alcohol-extracted crude | decreased plasma TAGs | rat | [126] | |
| decreased in plasma and hepatic TC | rat | [118,127] | ||
| enhanced cholesterol excretion; decreased TC levels | rabbit | [128] | ||
| decreased LDL | macaque | [129,130] | ||
| decreased serum TC and plasma LDL-C; increased plasma HDL-C | human | [118,119,127,131] | ||
| Globulins | hypocholesterolemic, hypolipidemic | [129,132] | ||
| hypocholesterolemic | rat | [133] | ||
| hypolipidemic | [103,116,133,134], | |||
| Conglycinin | anti-hypertension, anti-hypercholesterolemia, anti-dyslipidemia; reduced cholesterol absorption | [64,133,134,135,136,137,138,139,140,141] | ||
| decreased LDL and TC | cultured hepatocytes | [116,142,143,144,145] | ||
| decreased TC and TAGs | cultured hepatocytes | [142,146] | ||
| rat | [142] | |||
| decreased plasma TC and TAGs; improved LDL:HDL ratios | rat | [134] | ||
| decreased serum TAGs | mouse, human | [108,136,147] | ||
| decreased serum TAGs, fat, lipid accumulation and body:fat ratio | human | [100,148,149] | ||
| Conglycinin α’ | hypocholesterolemic | cultured hepatocytes | [116,145,146,150] | |
| decreased plasma TC and TAGs | rat | [135] | ||
| Lunasin | increased clearance of cholesterol | cultured hepatocytes | [151] | |
| increased clearance of hepatic and plasma LDL-C | pig | [151] | ||
| Lupinus spp. | Lupin | decreased plasma TC and non-HDL; increased HDL-C | hamster | [152] |
| decreased TAGs, TC, VLDL-C, LDL-C, VLDL-LDL ratio; increased HDL-C | rat | [153] | ||
| decreased plasma TAGs, TC, VLDL, LDL and liver cholesterol | rat | [154] | ||
| lowered cholesterol | rabbit, rat and human | [155,156,157,158,159] | ||
| improved LDL-HDL-C ratios | human | [160] | ||
| decreased blood pressure, LDL-C, LDL-HDL-C ratios | human | [157,160] | ||
| β-conglycinin | improved LDL-HDL-C ratios | cultured hepatocytes | [161] | |
| Conglutin-γ | hypocholesterolemic | [153] | ||
| decreased serum TC | mouse | [162] | ||
| Pea (Pisum sativum L.) | Crude | ACE inhibition | in vitro | [163] |
| decreased plasma TC and TAGs, hepatic TC; increased LDL-C | rat | [164,165] | ||
| decreased hepatic cholesterol and VLDL-C | rat | [166] | ||
| LRW peptide | Antioxidant; anti-inflammatory | in vitro and in muscle cells | [167] | |
| Cowpea (Vigna unguiculata L. Walp.) | Crude | ACE inhibition | in vitro | [13,168,169] |
| decreased plasma TC and non-HDL-C | hamster | [170] | ||
| 7S globulin | decreased liver TC and TAGs, serum non-HDL-C; atherogenic index; increased serum HDL-C | rat | [87] | |
| Jack bean (Canavalia ensiformis L.) | Crude | decreased TC and total lipids | rat | [171] |
| Concanavalin A | decreased serum TC, liver TC and TAGs; increased HDL-C | [172] | ||
| Mungbean (Vigna radiata L.) | Crude | decreased plasma TC, TAGs and non-HDL-C (non-HDL-cholesterol) | hamster | [173,174,175] |
| decreased TC, TAGs, non-HDL-C and TC:HDL-C ratio | rat | [176] | ||
| decreased systolic blood pressure and heart rate | rat | [173] | ||
| decreased TAGs visceral fat accumulation | human | [177,178] | ||
| Alcalase-hydrolyzed proteins | ACE inhibition, hypolipidemic, antihypertensive | rat | [173] | |
| Chickpea (Cicer aretinum L.) | Crude | decreased TC | cultured hepatocytes | [179] |
| decreased plasma TAGs, TC, LDL-C and VLDL, decreased liver TC and TAGs; plasma VLDL APOB-100, cholesterol and VLDL phospholipid levels; increased serum HDL-C and hepatic and fecal bile acids, TC and TAG | rat | [179,180,181,182] | ||
| decreased serum TC | human | [90,183,184] | ||
| Albumin | decreased serum TAGs, TC, LDL-C; increased HDL-C | mouse | [185] | |
| Lentil (Lens culinaris Medik.) | Crude | Decreased TC and LDL-C | rat | [186] |
| Convicillin | ACE inhibition | [187,188,189,190,191,192,193] | ||
| hyacinth bean (Lablab purpureus L.) | 7S globulin | ACE inhibition | in vitro | [14] |
| Moth bean (Phaseolus aconitifiolius Jacq.) | Crude | decreased plasma lipids, TC TAGs, LDL-C; decreased hepatic TC levels; increased HDL-C | rat | [194] |
| Faba bean (Vicia faba L.) | Crude | decrease of serum TC; LDL+HDL-C and atherogenic index; liver total and free cholesterols, TAGs and fat mass; increased excreted cholesterol | rat | [171] |
| Adzuki bean or red mungbean (Vicia angularis (Willd.) Ohwi and Ohashi) | 7S globulin | decreased serum TAGs, TC, non-HDL-C and atherogenic index; increased serum HDL-C; excreted total fat and TC levels | rat | [87] |
| Kidney beans (Phaseolus vulgaris L.) | Crude | decreased TC and LDL-C | human | [11] |
| Peanut (Arachis hypogaea L.) | Whole bean | decreased TC | human | [11] |
| GeneSet | p-Value | FDR | Inclusive Nodes |
|---|---|---|---|
| Fatty acid metabolic process | 1.69 × 10−11 | 4.21 × 10−9 | CPT1A,CPT1C,CPT1B,FASN,PPARG,PPARA |
| Regulation of cholesterol biosynthetic process | 3.35 × 10−10 | 4.16 × 10−8 | SREBF1,HMGCR,SREBF2,SCD,FASN |
| Regulation of lipid metabolic process | 6.96 × 10−10 | 5.77 × 10−8 | CPT1A,HMGCR,CYP7A1,RXRA,PPARG,PPARA |
| Response to retinoic acid | 1.59 × 10−9 | 9.83 × 10−8 | SREBF1,RXRB,RXRA,PPARG,RXRG |
| Steroid hormone mediated signaling pathway | 3.47 × 10−9 | 1.70 × 10−7 | RXRB,RXRA,PPARG,PPARA,RXRG |
| Response to lipid | 3.80 × 10−8 | 1.56 × 10−6 | SREBF1,SREBF2,PPARG, PPARA |
| Carnitine metabolic process | 6.02 × 10−8 | 2.11 × 10−6 | CPT1A,CPT1C,CPT1B |
| Lipid metabolic process | 1.67 × 10−7 | 5.16 × 10−6 | SREBF1,SREBF2,PPARG, PPARA,LDLR |
| Negative regulation of macrophage derived foam cell differentiation | 4.80 × 10−7 | 1.29 × 10−5 | CRP,PPARG,PPARA |
| Cellular response to low-density lipoprotein particle stimulus | 1.13 × 10−6 | 2.49 × 10−5 | SREBF2,PPARG,LDLR |
| Retinoic acid receptor signaling pathway | 1.13 × 10−6 | 2.49 × 10−5 | RXRB,RXRA,RXRG |
| Transcription initiation from RNA polymerase II promoter | 1.35 × 10−6 | 2.71 × 10−5 | RXRB,RXRA,PPARG,PPARA,RXRG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angeles, J.G.C.; Villanueva, J.C.; Uy, L.Y.C.; Mercado, S.M.Q.; Tsuchiya, M.C.L.; Lado, J.P.; Angelia, M.R.N.; Bercansil-Clemencia, M.C.M.; Estacio, M.A.C.; Torio, M.A.O. Legumes as Functional Food for Cardiovascular Disease. Appl. Sci. 2021, 11, 5475. https://doi.org/10.3390/app11125475
Angeles JGC, Villanueva JC, Uy LYC, Mercado SMQ, Tsuchiya MCL, Lado JP, Angelia MRN, Bercansil-Clemencia MCM, Estacio MAC, Torio MAO. Legumes as Functional Food for Cardiovascular Disease. Applied Sciences. 2021; 11(12):5475. https://doi.org/10.3390/app11125475
Chicago/Turabian StyleAngeles, Jorge Gil C., Jeric C. Villanueva, Lawrence Yves C. Uy, Sheila Mae Q. Mercado, Maria Claret L. Tsuchiya, Jickerson P. Lado, Mark Rickard N. Angelia, Mia Clare Marie Bercansil-Clemencia, Maria Amelita C. Estacio, and Mary Ann O. Torio. 2021. "Legumes as Functional Food for Cardiovascular Disease" Applied Sciences 11, no. 12: 5475. https://doi.org/10.3390/app11125475
APA StyleAngeles, J. G. C., Villanueva, J. C., Uy, L. Y. C., Mercado, S. M. Q., Tsuchiya, M. C. L., Lado, J. P., Angelia, M. R. N., Bercansil-Clemencia, M. C. M., Estacio, M. A. C., & Torio, M. A. O. (2021). Legumes as Functional Food for Cardiovascular Disease. Applied Sciences, 11(12), 5475. https://doi.org/10.3390/app11125475