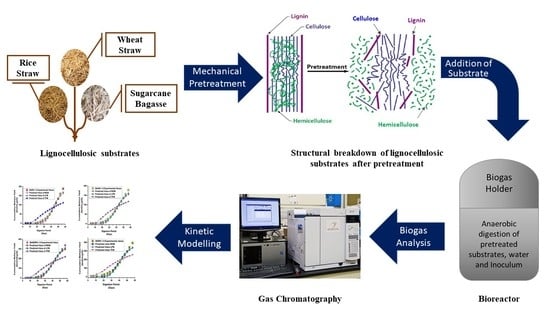
Enhanced Methane Production from Anaerobic Co-Digestion of Wheat Straw Rice Straw and Sugarcane Bagasse: A Kinetic Analysis
Abstract
:Highlights
- ⮚
- Mechanically pretreated wheat straw (WS), rice straw (RS), and sugarcane bagasse (BA)
- ⮚
- Anaerobic co-digestion of amalgamations
- ⮚
- Optimal substrate to inoculum ratio of 1.5 yield maximum biomethane
- ⮚
- Logistic function model best fit evaluated by using biokinetic parameters
1. Introduction
2. Material and Methods
2.1. Raw Material
2.2. Inoculum
2.3. Experimental Setup
2.4. Analytical Measurements
2.5. Kinetic Modeling
- (a)
- Logistic Function Model
- (b)
- Modified Gompertz Model
- (c)
- Transference Function Model
- (d)
- First Order Kinetics
3. Results and Discussion
3.1. Reactor Performance
3.2. Application of Kinetic Modelling
4. Way Forward
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Wei, Z.; Ji, Q.; Wang, C.; Gao, G. Global renewable energy development: Influencing factors, trend predictions and countermeasures. Resour. Policy 2019, 63. [Google Scholar] [CrossRef]
- Kamran, M. Current status and future success of renewable energy in Pakistan. Renew. Sustain. Energy Rev. 2018, 82, 609–617. [Google Scholar] [CrossRef]
- Ghafoor, A.; Rehman, T.U.; Munir, A.; Ahmad, M.; Iqbal, M. Current status and overview of renewable energy potential in Pakistan for continuous energy sustainability. Renew. Sustain. Energy Rev. 2016, 60, 1332–1342. [Google Scholar] [CrossRef]
- Boontian, N. Conditions of the Anaerobic Digestion of Biomass. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2014, 8, 1036–1040. [Google Scholar]
- Paul, S.; Dutta, A. Challenges and opportunities of lignocellulosic biomass for anaerobic digestion. Resour. Conserv. Recycl. 2018, 130, 164–174. [Google Scholar] [CrossRef]
- Wang, S.; Sun, X.; Yuan, Q. Strategies for enhancing microbial tolerance to inhibitors for biofuel production: A review. Bioresour. Technol. 2018, 258, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Gummert, M.; Van Hung, N.; Chivenge, P.; Douthwaite, B. Sustainable Rice Straw Management; Springer: New York, NY, USA, 2020. [Google Scholar]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, C. Bioresource Technology Anaerobic codigestion of pretreated wheat straw with cattle manure and analysis of the microbial community. Bioresour. Technol. 2015, 186, 128–135. [Google Scholar] [CrossRef]
- Rajput, A.A.; Sheikh, Z. Effect of inoculum type and organic loading on biogas production of sunflower meal and wheat straw. Sustain. Environ. Res. 2019, 29, 4. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Liu, S.; Mi, L.; Li, Z.; Yuan, Y.; Yan, Z.; Liu, X. Effects of feedstock ratio and organic loading rate on the anaerobic mesophilic co-digestion of rice straw and cow manure. Bioresour. Technol. 2015, 189, 319–326. [Google Scholar] [CrossRef]
- Soltanian, S.; Aghbashlo, M.; Almasi, F.; Hosseinzadeh-Bandbafha, H.; Nizami, A.-S.; Ok, Y.S.; Lam, S.S.; Tabatabei, M. A critical review of the effects of pretreatment methods on the exergetic aspects of lignocellulosic biofuels. Energy Convers. Manag. 2020, 212, 112792. [Google Scholar] [CrossRef]
- Zealand, A.; Roskilly, A.; Graham, D. Effect of feeding frequency and organic loading rate on biomethane production in the anaerobic digestion of rice straw. Appl. Energy 2017, 207, 156–165. [Google Scholar] [CrossRef]
- Ríos-Badrán, I.M.; Luzardo-Ocampo, I.; García-Trejo, J.F.; Santos-Cruz, J.; Gutiérrez-Antonio, C. Production and characterization of fuel pellets from rice husk and wheat straw. Renew. Energy 2020, 145, 500–507. [Google Scholar] [CrossRef]
- Moretti, M.M.D.S.; Perrone, O.M.; Nunes, C.D.C.C.; Taboga, S.; Boscolo, M.; Da Silva, R.; Gomes, E. Effect of pretreatment and enzymatic hydrolysis on the physical-chemical composition and morphologic structure of sugarcane bagasse and sugarcane straw. Bioresour. Technol. 2016, 219, 773–777. [Google Scholar] [CrossRef]
- Yao, Y.; Bergeron, A.D.; Davaritouchaee, M. Methane recovery from anaerobic digestion of urea-pretreated wheat straw. Renew. Energy 2018, 115, 139–148. [Google Scholar] [CrossRef]
- Hobbs, S.R.; Landis, A.E.; Rittmann, B.E.; Young, M.N.; Parameswaran, P. Enhancing anaerobic digestion of food waste through biochemical methane potential assays at different substrate: Inoculum ratios. Waste Manag. 2018, 71, 612–617. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, T.; Dong, C.-Q.; Lu, Q.; Ali, Z.; Khan, I.; Hussain, Z.; Abbas, A. Sketching Pakistan’s energy dynamics: Prospects of biomass energy. J. Renew. Sustain. Energy 2018, 10, 023101. [Google Scholar] [CrossRef]
- Saratale, G.D.; Jung, M.-Y.; Oh, M.-K. Reutilization of green liquor chemicals for pretreatment of whole rice waste biomass and its application to 2,3-butanediol production. Bioresour. Technol. 2016, 205, 90–96. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Varjani, S.; Cho, S.-K.; Ghodake, G.S.; Kadam, A.; I Mulla, S.; Bharagava, R.N.; Kim, D.-S.; Shin, H.S. Development of ultrasound aided chemical pretreatment methods to enrich saccharification of wheat waste biomass for polyhydroxybutyrate production and its characterization. Ind. Crop. Prod. 2020, 150, 112425. [Google Scholar] [CrossRef]
- Belle, A.J.; Lansing, S.; Mulbry, W.; Weil, R.R. Methane and hydrogen sulfide production during co-digestion of forage radish and dairy manure. Biomass Bioenergy 2015, 80, 44–51. [Google Scholar] [CrossRef]
- Reungsang, A.; Pattra, S.; Sittijunda, S. Optimization of Key Factors Affecting Methane Production from Acidic Effluent Coming from the Sugarcane Juice Hydrogen Fermentation Process. Energies 2012, 5, 4746–4757. [Google Scholar] [CrossRef] [Green Version]
- Karray, R.; Karray, F.; Loukil, S.; Mhiri, N.; Sayadi, S. Anaerobic co-digestion of Tunisian green macroalgae Ulva rigida with sugar industry wastewater for biogas and methane production enhancement. Waste Manag. 2017, 61, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Telliard, W.A. METHOD 1684 Total, Fixed, and Volatile Solids in Water, Solids, and Biosolids; US Environmental Protection Agency: Washington, DC, USA, 2001.
- APHA/AWWA/WEF. Standard Methods for Examination of Water and Wastewater. Stand. Methods. 2012, p. 541. Available online: https://www.wef.org/resources/publications/books/StandardMethods/ (accessed on 31 May 2021).
- Al-Selwi, A.; Joshi, M. Source Rock Evaluation using Total Organic Carbon (TOC) and the Loss- On-Ignition (LOI) Techniques. Oil Gas Res. 2015, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Nie, H.; Jacobi, H.F.; Strach, K.; Xu, C.; Zhou, H.; Liebetrau, J. Mono-fermentation of chicken manure: Ammonia inhibition and recirculation of the digestate. Bioresour. Technol. 2015, 178, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Nguimkeu, P. A simple selection test between the Gompertz and Logistic growth models. Technol. Forecast. Soc. Chang. 2014, 88, 98–105. [Google Scholar] [CrossRef]
- Chauvatcharin, S.; Siripatana, C.; Seki, T.; Takagi, M.; Yoshida, T. Metabolism analysis and on-line physiological state diagnosis of acetone–butanol fermentation. Biotechnol. Bioeng. 1998, 58, 561–571. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. Enhanced methane production from anaerobic co-digestion of rice straw and hydrilla verticillata and its kinetic analysis. Biomass Bioenergy 2019, 125, 8–16. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Surendra, K.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef]
- Kumar, G.; Dharmaraja, J.; Arvindnarayan, S.; Shoban, S.; Bakonyi, P.; Saratale, G.D.; Nemestóthy, N.; Bélafi–Bakó, K.; Yoon, J.; Kim, S. A comprehensive review on thermochemical, biological, biochemical and hybrid conversion methods of bio-derived lignocellulosic molecules into renewable fuels. Fuel 2019, 251, 352–367. [Google Scholar] [CrossRef]
- Hassan, M.; Ding, W.; Shi, Z.; Zhao, S. Methane enhancement through co-digestion of chicken manure and thermo-oxidative cleaved wheat straw with waste activated sludge: A C/N optimization case. Bioresour. Technol. 2016, 211, 534–541. [Google Scholar] [CrossRef]
- Liew, L.N.; Shi, J.; Li, Y. Enhancing the solid-state anaerobic digestion of fallen leaves through simultaneous alkaline treatment. Bioresour. Technol. 2011, 102, 8828–8834. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.; Wang, H.; Rehman, S.; Dong, C.; Hsu, H.-Y.; Lin, C.S.K.; Leu, S.-Y. Sustainability metrics of pretreatment processes in a waste derived lignocellulosic biomass biorefinery. Bioresour. Technol. 2020, 298, 122558. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, H.; Moniruzzaman, M.; Iqbal, T.; Khan, M.J. Recent advances in the pretreatment of lignocellulosic biomass for biofuels and value-added products. Curr. Opin. Green Sustain. Chem. 2019, 20, 18–24. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Syaichurrozi, I. Biogas production from co-digestion Salvinia molesta and rice straw and kinetics. Renew. Energy 2018, 115, 76–86. [Google Scholar] [CrossRef]
- Zou, H.; Chen, Y.; Shi, J.; Zhao, T.; Yu, Q.; Yu, S.; Shi, D.; Chai, H.; Gu, L.; He, Q.; et al. Mesophilic anaerobic co-digestion of residual sludge with different lignocellulosic wastes in the batch digester. Bioresour. Technol. 2018, 268, 371–381. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Veluchamy, C.; Kalamdhad, A.S. Biochemical methane potential test for pulp and paper mill sludge with different food/microorganisms ratios and its kinetics. Int. Biodeterior. Biodegrad. 2017, 117, 197–204. [Google Scholar] [CrossRef]
- Eryildiz, B.; Lukitawesa; Taherzadeh, M.J. Effect of pH, substrate loading, oxygen, and methanogens inhibitors on volatile fatty acid (VFA) production from citrus waste by anaerobic digestion. Bioresour. Technol. 2020, 302, 122800. [Google Scholar] [CrossRef]
- Dinamarca, S.; Aroca, G.; Chamy, R.; Guerrero, L. The influence of pH in the hydrolytic stage of anaerobic digestion of the organic fraction of urban solid waste. Water Sci. Technol. 2003, 48, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Wu, Z.; Shrestha, S.; Lee, P.-H.; Raskin, L.; Khanal, S.K. Intermittent micro-aeration: New strategy to control volatile fatty acid accumulation in high organic loading anaerobic digestion. Water Res. 2019, 166, 115080. [Google Scholar] [CrossRef] [PubMed]
- Kiran, E.U.; Stamatelatou, K.; Antonopoulou, G.; Lyberatos, G. Production of Biogas via Anaerobic Digestion; Elsevier: Amsterdam, The Netherlands, 2016; pp. 259–301. [Google Scholar]





| Parameters | Wheat Straw | Rice Straw | Bagasse | Inoculum |
|---|---|---|---|---|
| Total Solids % | 99 | 98.5 | 98 | 85 |
| Volatile Solids % | 83.33 | 62.42 | 80 | 58.6 |
| Total Organic Carbon (TOC) % | 46.29 | 34.46 | 44.44 | 32.555 |
| Moisture Content % | 1 | 1.5 | 2 | 90.6 |
| pH | - | - | - | 6.6 |
| Reactors | pH | VS Reduction (%) |
|---|---|---|
| Control 1 | 7.0 | 88.9 |
| WSRS | 7.0 | 98.8 |
| BARS | 6.9 | 98.5 |
| BAWS | 7.8 | 98.1 |
| BAWSRS | 7.0 | 99.0 |
| Reactors | pH | VS Reduction (%) |
|---|---|---|
| Control 2 | 7.1 | 87.0 |
| WSRS | 7.1 | 99.2 |
| BARS | 5.5 | 99.4 |
| BAWS | 6.5 | 97.7 |
| BAWSRS | 7.0 | 96.9 |
| Parameters | S/I 1.5 | S/I 2.5 | ||||||
|---|---|---|---|---|---|---|---|---|
| WSRS | BAWS | BARS | BAWSRS | WSRS | BAWS | BARS | BAWSRS | |
| Modified Gompertz Model | ||||||||
| R2 | 0.972 | 0.977 | 0.977 | 0.981 | 0.981 | 0.983 | 0.975 | 0.984 |
| RMSE | 0.581 | 0.269 | 0.638 | 0.541 | 0.594 | 0.682 | 0.638 | 0.636 |
| λ (days) | 10 | 18 | 22 | 17 | 18 | 25 | 22 | 21 |
| Rm mLCH4/VS. d | 4.123 | 0.300 | 0.256 | 0.501 | 0.298 | 0.091 | 0.0181 | 0.1574 |
| Predicted Methane Yield NmLCH4/gVS | 338.878 | 154.768 | 163.010 | 295.993 | 215.493 | 57.732 | 13.560 | 133.583 |
| Experimental Methane NmLCH4/gVS | 393.008 | 177.965 | 188.299 | 337.900 | 244.785 | 65.793 | 15.748 | 151.345 |
| Difference % (ϓ) | 6.8 | 7.21 | 4.84 | 6.23 | 5.86 | 5.63 | 7.44 | 5.82 |
| Logistic Function Model | ||||||||
| R2 | 0.990 | 0.992 | 0.991 | 0.993 | 0.994 | 0.992 | 0.990 | 0.994 |
| RMSE | 0.138 | 0.23 | 0.115 | 0.166 | 0.303 | 0.682 | 0.656 | 0.200 |
| λ (days) | 10 | 17 | 21 | 15 | 19 | 36 | 33 | 25 |
| Rm mLCH4/VS. d | 2.566 | 1.477 | 2.368 | 2.713 | 1.260 | 0.670 | 0.191 | 2.002 |
| Predicted Methane Yield NmLCH4/gVS | 335.716 | 161.425 | 170.274 | 66.334 | 224.178 | 59.895 | 14.151 | 38.107 |
| Experimental Methane Yield NmLCH4/gVS | 393.008 | 177.965 | 188.299 | 337.900 | 244.785 | 65.793 | 15.748 | 41.399 |
| Difference % (ϓ) | 4.67 | 5.39 | 4.39 | 4.68 | 4.04 | 4.12 | 4.91 | 4.14 |
| Transfer Function Model | ||||||||
| R2 | 0.737 | 0.742 | 0.677 | 0.744 | 0.685 | 0.575 | 0.642 | 0.641 |
| RMSE | 0.660 | 2.456 | 3.453 | 3.659 | 1.965 | 10.293 | 3.66 | 4.141 |
| (days) | 11 | 15 | 13 | 11 | 12 | 14 | 13 | 12 |
| Rm | 2.566 | 0.164 | 1.106 | 1.757 | 1.260 | 0.212 | 0.080 | 0.831 |
| Predicted Methane Yield mLCH4/gVS | 252.851 | 116.326 | 111.758 | 219.783 | 150.791 | 34.445 | 8.930 | 87.913 |
| Experimental Methane Yield NmLCH4/gVS | 393.008 | 177.965 | 188.299 | 337.900 | 244.785 | 65.793 | 15.748 | 151.345 |
| Difference % (ϓ) | 21.18 | 20.9 | 25.47 | 19.74 | 23.03 | 30.62 | 26.99 | 26.06 |
| First Order Kinetics | ||||||||
| Kh | 0.03 | 0.02 | 0.007 | 0.03 | 0.019 | 0.009 | 0.005 | 0.009 |
| Predicted Methane Yield NmLCH4/gVS | 334.206 | 140.3248 | 52.3473 | 284.856 | 163.671 | 52.576 | 10.251 | 100.486 |
| Experimental Methane Yield NmLCH4/gVS | 393.008 | 177.965 | 188.299 | 337.900 | 244.785 | 65.793 | 15.748 | 151.345 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meraj, S.; Liaquat, R.; Raza Naqvi, S.; Sheikh, Z.; Zainab, A.; Khoja, A.H.; Juchelkova, D.; Atabani, A. Enhanced Methane Production from Anaerobic Co-Digestion of Wheat Straw Rice Straw and Sugarcane Bagasse: A Kinetic Analysis. Appl. Sci. 2021, 11, 6069. https://doi.org/10.3390/app11136069
Meraj S, Liaquat R, Raza Naqvi S, Sheikh Z, Zainab A, Khoja AH, Juchelkova D, Atabani A. Enhanced Methane Production from Anaerobic Co-Digestion of Wheat Straw Rice Straw and Sugarcane Bagasse: A Kinetic Analysis. Applied Sciences. 2021; 11(13):6069. https://doi.org/10.3390/app11136069
Chicago/Turabian StyleMeraj, Saadia, Rabia Liaquat, Salman Raza Naqvi, Zeshan Sheikh, Atoofa Zainab, Asif Hussain Khoja, Dagmar Juchelkova, and Abdulaziz Atabani. 2021. "Enhanced Methane Production from Anaerobic Co-Digestion of Wheat Straw Rice Straw and Sugarcane Bagasse: A Kinetic Analysis" Applied Sciences 11, no. 13: 6069. https://doi.org/10.3390/app11136069
APA StyleMeraj, S., Liaquat, R., Raza Naqvi, S., Sheikh, Z., Zainab, A., Khoja, A. H., Juchelkova, D., & Atabani, A. (2021). Enhanced Methane Production from Anaerobic Co-Digestion of Wheat Straw Rice Straw and Sugarcane Bagasse: A Kinetic Analysis. Applied Sciences, 11(13), 6069. https://doi.org/10.3390/app11136069