A Synergistic Anti-Diabetic Effect by Ginsenosides Rb1 and Rg3 through Adipogenic and Insulin Signaling Pathways in 3T3-L1 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Line and Materials
2.2. Cell Culture
2.3. Determination of Lipid Accumulation by Oil Red O Staining
2.4. RNA Analysis by Semi-Quantitative Reverse Transcriptase Polymerase Reaction (RT-PCR)
2.5. Statistical Analysis
3. Results
3.1. Ginsenosides Rb1 and Rg3 Enhanced the Differentiation of 3T3-L1 Preadipocytes
3.2. Ginsenosides Rb1 and Rg3 Upregulate GLUT4 and Adiponectin mRNA Expression
3.3. Ginsenosides Rb1 and Rg3 Increase mRNA Expression of the Glucose Transport System
3.4. Combined Effects of Ginsenosides Rb1 and Rg3 on the Expression of GLUT4 and Adiponectin mRNAs
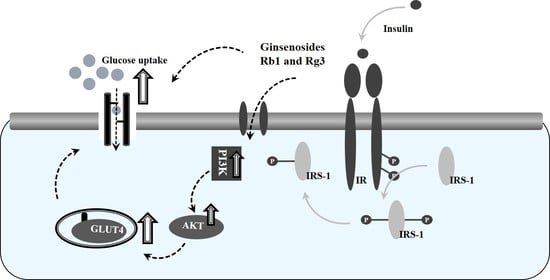
3.5. Combined Effects of Ginsenosides Rb1 and Rg3 on the Insulin Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; Forge, R.L.; et al. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefan, N.; Kantartzis, K.; Machann, J.; Schick, F.; Thamer, C.; Rittig, K.; Balletshofer, B.; Machicao, F.; Fritsche, A.; Häring, H. Identification and characterization of metabolically benign obesity in humans. Arch. Intern. Med. 2008, 168, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.A.; Succurro, E.; Frontoni, S.; Hribal, M.L.; Andreozzi, F.; Lauro, R.; Perticone, F.; Sesti, G. Metabolically healthy but obese women have an intermediate cardiovascular risk profile between healthy nonobese women and obese insulin-resistant women. Diabetes Care 2007, 30, 2145–2147. [Google Scholar]
- Lafontan, M. Adipose tissue and adipocyte dysregulation. Diabetes Metab. 2014, 40, 16–28. [Google Scholar] [CrossRef]
- Cignarelli, A.; Genchi, V.A.; Perrini, S.; Natalicchio, A.; Laviola, L.; Giorgino, F. Insulin and Insulin Receptors in Adipose Tissue Development. Int. J. Mol. Sci. 2019, 20, 759. [Google Scholar] [CrossRef] [Green Version]
- Isakson, P.; Hammarstedt, A.; Gustafson, B.; Smith, U. Impaired preadipocyte differentiation in human abdominal obesity: Role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes 2009, 58, 1550–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Jiménez, V.; Cortez-Espinosa, N.; Rodríguez-Varela, E.; Vega-Cárdenas, M.; Briones-Espinoza, M.; Ruíz-Rodríguez, V.M.; López-López, N.; Briseño-Medina, A.; Turiján-Espinoza, E.; Portales-Pérez, D.P. Altered levels of sirtuin genes (SIRT1, SIRT2, SIRT3 and SIRT6) and their target genes in adipose tissue from individual with obesity. Diabetes Metab. Syndr. 2019, 13, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996, 271, 665–670. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Arushanian, E.B. Therapeutic potential of ginseng root preparations in treating diabetes mellitus. Eksp. Klin. Farmakol. 2009, 72, 52–56. [Google Scholar]
- Bang, H.; Kwak, J.H.; Ahn, H.Y.; Shin, D.Y.; Lee, J.H. Korean red ginseng improves glucose control in subjects with impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes mellitus. J. Med. Food 2014, 17, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.W.; Kim, Y.C.; Rhee, Y.K.; Lee, Y.C.; Kim, K.T.; Hong, H.D. Chemical composition characteristics of Korean straight ginseng products. J. Ethn. Foods 2014, 1, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Zhao, S. Progress in understanding of ginsenoside biosynthesis. Plant. Biol. 2008, 10, 415–421. [Google Scholar] [CrossRef]
- Kim, D.H. Chemical Diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J. Ginseng Res. 2012, 36, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.Y.; Kim, J.M.; Han, S.B.; Lee, S.K.; Kim, N.D.; Park, M.K.; Kim, C.K.; Park, J.H. Steaming of ginseng at high temperature enhances biological activity. J. Nat. Prod. 2000, 63, 1702–1704. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, B.; Song, W.; Wang, A.; Ni, M.; Luo, X.; Aung, H.H.; Xie, J.; Tong, R.; He, T.; et al. Steamed American ginseng berry: Ginsenoside analyses and anticancer activities. J. Agric. Food Chem. 2006, 54, 9936–9942. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.Y.; Moon, J.M.; Kim, B.Y.; Lim, S.H.; Lee, G.S.; Yu, H.S.; Cho, S.I. Comparative study of Korean white ginseng and Korean Red Ginseng on efficacies of ova-induced asthma model in mice. J. Ginseng Res. 2015, 39, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Shibata, S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J. Korean Med. Sci. 2001, 16, 28–37. [Google Scholar]
- He, M.; Huang, X.; Liu, S.; Guo, C.; Xie, Y.; Meijer, A.H.; Wang, M. The Difference between White and Red Ginseng: Variations in Ginsenosides and Immunomodulation. Planta Med. 2018, 84, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, Y.W.; Lim, S.S.; Ha, I.J.; Na, Y.C.; Seo, J.J.; Shin, H.; Son, S.H.; Kim, Y.S. Preparative isolation of four ginsenosides from Korean red ginseng (steam-treated Panax ginseng C. A. Meyer), by high-speed counter-current chromatography coupled with evaporative light scattering detection. J. Chromatogr. A 2007, 1151, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.W.; Han, S.B.; Park, I.H.; Kim, J.M.; Park, M.K.; Park, J.H. Liquid chromatographic determination of less polar ginsenosides in processed ginseng. J. Chromatogr. A 2001, 921, 335–339. [Google Scholar] [CrossRef]
- Cho, H.T.; Kim, J.H.; Lee, J.H.; Kim, Y.J. Effects of Panax ginseng extracts prepared at different steaming times on thermogenesis in rats. J. Ginseng Res. 2017, 41, 347–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Ji, G.E. Effects of various ginsenosides and ginseng root and ginseng berry on the activity of pancreatic lipase. Food Sci. Biotechnol. 2017, 26, 767–773. [Google Scholar] [CrossRef]
- Hasegawa, H. Proof of the mysterious efficacy of ginseng: Basic and clinical trials: Metabolic activation of ginsenoside: Deglycosylation by intestinal bacteria and esterification with fatty acid. J. Pharmacol. Sci. 2004, 95, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Kida, T.; Kanaoka, M.; Hattori, M.; Kobashi, K. Drug metabolism: Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J. Pharm. Pharmacol. 1998, 50, 1155–1160. [Google Scholar]
- Bae, E.A.; Park, S.Y.; Kim, D.H. Constitutive β-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol. Pharm. Bull. 2000, 23, 1481–1485. [Google Scholar] [CrossRef]
- Yun, S.N.; Moon, S.J.; Ko, S.K. Wild ginseng prevents the onset of high fat diet induced hyperglyecemia and obesity in ICR mice. Arch. Pharm. Res. 2004, 27, 790–796. [Google Scholar] [PubMed]
- Chung, S.H.; Choi, C.G.; Park, S.H. Comparisons between white ginseng radix and rootlet for antidiabetic activity and mechanism in KKAy mice. Arch. Pharm. Res. 2001, 24, 214–218. [Google Scholar] [CrossRef]
- Shang, W.; Yang, Y.; Zhou, L.; Jiang, B.; Jin, H.; Chen, M. Ginsenoside Rb1 stimulates glucose uptake through insulin-like signaling pathway in 3T3-L1 adipocytes. J. Endocrinol. 2008, 198, 561. [Google Scholar] [CrossRef]
- Hwang, J.T.; Lee, M.S.; Kim, H.J.; Sung, M.J.; Kim, H.Y.; Kim, M.S.; Kwon, D.Y. Antiobesity effect of ginsenoside Rg3 involves the AMPK and PPAR-gamma signal pathways. Phytother. Res. 2009, 23, 262–266. [Google Scholar]
- Mohanan, P.; Subramaniyam, S.; Mathiyalagan, R.; Yang, D.C. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J. Ginseng Res. 2018, 42, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Park, M.W.; Ha, J.; Chung, S.H. 20(S)-ginsenoside Rg3 enhances glucose-stimulated insulin secretion and activates AMPK. Biol. Pharm. Bull. 2008, 31, 748–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.; Yoon, J.W.; Choi, S.H.; Cho, B.J.; Kim, J.T.; Chang, H.S.; Park, H.S.; Park, K.S.; Lee, H.K.; Kim, Y.B.; et al. Effect of ginsam, a vinegar extract from Panax ginseng, on body weight and glucose homeostasis in an obese insulin-resistant rat model. Metabolism 2009, 58, 8–15. [Google Scholar] [CrossRef]
- Kim, S.N.; Lee, J.H.; Shin, H.; Son, S.H.; Kim, Y.S. Effects of in vitro digested ginsenosides on lipid accumulation in 3T3-L1 adipocytes. Planta. Med. 2009, 75, 596–601. [Google Scholar] [CrossRef]
- Hwang, J.T.; Kim, S.H.; Lee, M.S.; Kim, S.H.; Yang, H.J.; Kim, M.J.; Kim, H.S.; Ha, J.; Kim, M.S.; Kwon, D.Y. Anti-obesity effects of ginsenoside Rh2 are associated with the activation of AMPK signaling pathway in 3T3-L1 adipocyte. Biochem. Biophys. Res. Commun. 2007, 364, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Yang, Y.; Jiang, B.; Jin, H.; Zhou, L.; Liu, S.; Chen, M. Ginsenoside Rb1 promotes adipogenesis in 3T3-L1 cells by enhancing PPARγ2 and C/EBPα gene expression. Life Sci. 2007, 80, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.S.; Yue, P.Y.; Kok, T.W.; Keung, M.H.; Mak, N.K.; Wong, R.N. Ginsenoside-Rb1 promotes adipogenesis through regulation of PPARγ and microRNA-27b. Horm. Metab. Res. 2012, 44, 819–824. [Google Scholar] [CrossRef]
- Han, K.L.; Jung, M.H.; Sohn, H.H.; Hwang, J.K. Ginsenoside 20(S)-protopanaxatriol (PPT) activates peroxisome proliferator-activated receptor gamma (PPAR gamma) in 3T3-L1 adipocytes. Biol. Pharm. Bull. 2006, 29, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Blumberg, J.M.; Tzameli, I.; Astapova, I.; Lam, F.S.; Flier, J.S.; Hollenberg, A.N. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. J. Biol. Chem. 2006, 28, 11205–11213. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, G.; Sun, J.; Hao, H.; Xiong, Y.; Yan, B.; Zheng, Y.; Sheng, L. Pharmacokinetic and absolute bioavailability study of total panax notoginsenoside, a typical multiple constituent traditional Chinese medicine (TCM) in rats. Biol. Pharm. Bull. 2007, 30, 847–851. [Google Scholar]
- Xie, H.T.; Wang, G.J.; Sun, J.G.; Tucker, I.; Zhao, X.C.; Xie, Y.Y.; Li, H.; Jiang, X.L.; Wang, R.; Xu, M.J.; et al. High performance liquid chromatographic-mass spectrometric determination of ginsenoside Rg3 and its metabolites in rat plasma using solid-phase extraction for pharmacokinetic studies. J. Chromatogr. B 2005, 818, 167–173. [Google Scholar] [CrossRef]
- Kim, H.K. Pharmacokinetics of ginsenoside Rb1 and its metabolite compound K after oral administration of Korean Red Ginseng extract. J. Ginseng Res. 2013, 37, 451. [Google Scholar] [PubMed] [Green Version]
- Olefsky, J.M.; Ciaraldi, T.P.; Kolterman, O.G. Mechanisms ofinsulin resistance in non-insulin-dependent (type II) diabetes. Am. J. Med. 1985, 79, 12–22. [Google Scholar] [CrossRef]
- Pandeti, S.; Arha, D.; Mishra, A.; Reddy, S.S.; Srivastava, A.K.; Narender, T.; Tamrakar, A.K. Glucose uptake stimulatory potential and antidiabetic activity of the Arnebin-1 from Arnabia nobelis. Eur. J. Pharmacol. 2016, 789, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Schlegel, A.; Scherer, P.E.; Lisanti, M.P. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J. Biol. Chem. 1998, 273, 5419–5422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.Q.; Na, J.R.; Bang, M.H.; Kim, M.K.; Yang, D.C. Conversion of major ginsenoside Rb1 to 20(S)-ginsenoside Rg3 by Microbacterium sp. GS514. Phytochemistry 2008, 69, 218–224. [Google Scholar]
- Lee, W.K.; Kao, S.T.; Liu, L.M.; Cheng, J.T. Ginsenoside Rh2 is one of the active principles of Panax ginseng root to improve insulin sensitivity in fructose-rich chow-fed rats. Horm. Metab. Res. 2007, 39, 347–354. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Review of Ginseng Anti-Diabetic Studies. Molecules 2019, 24, 4501. [Google Scholar] [CrossRef] [Green Version]
- Ahima, R.S. Metabolic actions of adipocyte hormones: Focus on adiponectin. Obesity 2006, 14, 9–15. [Google Scholar] [CrossRef]
- Maeda, N.; Shimomura, I.; Kishida, K.; Nishizawa, H.; Matsuda, M.; Nagaretani, H.; Furuyama, N.; Kondo, H.; Takahashi, M.; Arita, Y.; et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002, 8, 731–737. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawrocki, A.R.; Rajala, M.W.; Tomas, E.; Pajvani, U.B.; Saha, A.K.; Trumbauer, M.E.; Pang, Z.; Chen, A.S.; Ruderman, N.B.; Chen, H.; et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J. Biol. Chem. 2006, 281, 2654–2660. [Google Scholar] [CrossRef] [Green Version]
- Kubota, N.; Terauchi, Y.; Kubota, T.; Kumagai, H.; Itoh, S.; Satoh, H.; Yano, W.; Ogata, H.; Tokuyama, K.; Takamoto, I.; et al. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. J. Biol. Chem. 2006, 281, 8748–8755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staels, B.; Fruchart, J.C. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes 2005, 54, 2460–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.T.; Wang, C.Z.; Wang, A.B.; Wu, J.; Basila, D.; Yuan, C.S. Antihyperglycemic effects of total ginsenosides from leaves and stem of Panax ginseng. Acta Pharmacol. Sin. 2005, 26, 1104–1110. [Google Scholar] [CrossRef] [Green Version]
- Furtado, L.M.; Somwar, R.; Sweeney, G.; Niu, W.; Klip, A. Activation of the glucose transporter GLUT4 by insulin. Biochem. Cell Biol. 2002, 80, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xie, Y.; Morrison, R.F.; Bucher, N.L.; Farmer, S.R. PPARgamma induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPalpha during the conversion of 3T3 fibroblasts into adipocytes. J. Clin. Investig. 1998, 101, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Ahn, B.Y.; Lee, J.S.; Chung, S.S.; Lim, S.; Park, S.G.; Jung, H.S.; Lee, H.K.; Park, K.S. The ginsenoside Rg3 has a stimulatory effect on insulin signaling in L6 myotubes. Biochem. Biophys. Res. Commun. 2009, 389, 70–73. [Google Scholar] [CrossRef]
- Baeg, I.H.; Seo, S.H. The world ginseng market and the ginseng (Korea). J. Ginseng Res. 2013, 37, 1–7. [Google Scholar] [CrossRef] [Green Version]






| Primer Sequences (5′→3′) | ||
|---|---|---|
| PPARγ | Forward | CCA GAG TCT GCT GAT CTG CG |
| Reverse | GCC ACC TCT TTG CTC TGA TC | |
| C/EBP α | Forward | GGT GCG CAA GAG CCG AGA TAA AG |
| Reverse | AGT TCA CGG CTC AGC TGT TCC AC | |
| aP2 | Forward | GAC CTG GAA ACT CGT CTC CA |
| Reverse | CAT GAC ACA TTC CAC CAC CA | |
| GLUT4 | Forward | TAC TCA TTC TTG GAC GGT TC |
| Reverse | TGA TGT AGA GGT ATC TGG GG | |
| adiponectin | Forward | AAG GAC AAG GCCG TTC TCT |
| Reverse | TAT GGG TAG TTG CAG TCA GTT GG | |
| IRS-1 | Forward | TCG TCA ATA GCG TAA CTG GA |
| Reverse | AGA ACG TGC AGT TCA GTC AA | |
| PI3K | Forward | CAT GTT CTG GAA ACT TCA CCA |
| Reverse | CCT GGG GAA ACA TAA ACT TG | |
| Akt | Forward | AGA GAT GGG GAT AGG TGT CT |
| Reverse | ACT CAC ACA AGT CTG CAT A | |
| β-actin | Forward | AGG CTG TGC TGT CCC TGT ATG C |
| Reverse | ACC CAAG AAG GAA GGC TGG AAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, H.-D.; Choi, S.-I.; Lee, O.-H.; Kim, Y.-C. A Synergistic Anti-Diabetic Effect by Ginsenosides Rb1 and Rg3 through Adipogenic and Insulin Signaling Pathways in 3T3-L1 Cells. Appl. Sci. 2021, 11, 1725. https://doi.org/10.3390/app11041725
Hong H-D, Choi S-I, Lee O-H, Kim Y-C. A Synergistic Anti-Diabetic Effect by Ginsenosides Rb1 and Rg3 through Adipogenic and Insulin Signaling Pathways in 3T3-L1 Cells. Applied Sciences. 2021; 11(4):1725. https://doi.org/10.3390/app11041725
Chicago/Turabian StyleHong, Hee-Do, Sun-Il Choi, Ok-Hwan Lee, and Young-Cheul Kim. 2021. "A Synergistic Anti-Diabetic Effect by Ginsenosides Rb1 and Rg3 through Adipogenic and Insulin Signaling Pathways in 3T3-L1 Cells" Applied Sciences 11, no. 4: 1725. https://doi.org/10.3390/app11041725
APA StyleHong, H. -D., Choi, S. -I., Lee, O. -H., & Kim, Y. -C. (2021). A Synergistic Anti-Diabetic Effect by Ginsenosides Rb1 and Rg3 through Adipogenic and Insulin Signaling Pathways in 3T3-L1 Cells. Applied Sciences, 11(4), 1725. https://doi.org/10.3390/app11041725