1. Introduction
As a sensory organ, the nose is physiologically complex and anatomically variegated by its nature. Through our breathing organs, the nose primes the flow of air, which is humidified, heated and filtered right inside. Remarkably, the olfactory function is ascribed to this organ [
1]. From an anatomical point of view, different tissue types are present in the nose including cartilage, bone, fibrous, muscle, adipose, and epithelial, which are structurally interconnected with each other through ligaments of different thickness. The integrity of all the anatomical components is essential for the respiratory function [
2,
3,
4].
The cartilaginous component, of the hyaline type, gives rise to the septum that divides the nose into two cavities; it also constitutes both upper and lower lateral cartilages [
5]. The nasal septum acts as a support for the other nose structures to confer rigidity, thus preventing its permanent deformation. Through the lateral cartilages, the nose interfaces with the nasal bones, such as the vomer, the perpendicular plate of ethmoid bone, and incisive bone, which determine nose anchoring to the facial structures [
4,
6]. Proceeding from the surface to the depth of the nose, a series of layers are present, made up of different tissues devoted to stability and protection against mechanical shocks along with ensuring the correct organ function: the adipose panniculus, the fibromuscular layer rich in vessels, muscles and ligaments, the deep adipose layer and the muco-perichondrium [
7,
8,
9]. Furthermore, two fibrocartilaginous sacs have recently been identified, one right and one left, linked to the development of nasal cartilages and containing, in their walls, the olfactory mucosa [
3].
Given its filtering function and its position, the nose is exposed to a series of injuries of traumatic, chemical, inflammatory and tumorigenic origin [
4]. The most common injuries are septal deformations, which cause obstructions of the airways. In addition, nose is subject to tumors, rare but extremely variegated in nature, whose resection causes profound alterations in the cartilaginous structures. Surgery is necessary to repair the damaged anatomical tissues, which, depending on the injury, may require subsequent revisions [
10,
11]. The surgical procedures currently employed are rhinoplasty and septoplasty, which often exploit cartilaginous grafts to repair damaged structures [
2]. The gold standard is autologous cartilage obtained from the septum itself, the auricle or the ribs. The first site is easily accessible, but the amount that can be recovered is limited; on the contrary, plenty of material can be taken from the ribs, but the removal procedure causes donor site morbidity [
12,
13,
14]. As an alternative, it is possible to use allogeneic grafts. Even though their actual immunogenicity is not known, the limited cellular phase present in cartilage suggests a limited risk [
15,
16]. Finally, it is possible to apply synthetic materials, which can be molded into the desired shapes, they are cheap and implantable in a single procedure; however, they can lead to sustained inflammation up to device extrusion [
17,
18]. Thus, an effective and simple reconstructive procedure with minimal side effects has yet to be developed.
A novel approach to nasal reconstruction is shown by tissue engineering (TE). Some studies have proposed biocompatible scaffolds based on decellularized cartilage, collagen, polycaprolactone (PCL), poly(L-lactic) acid (PLLA), poly (lactic-co-glycolic) acid (PLGA) [
19,
20,
21,
22], and stem cells, such as mesenchymal stromal cells (MSCs), easy to isolate and already used in clinical procedures, or septal chondrocytes [
23,
24,
25]. Injectable fibrin scaffolds containing septal chondrocytes have been applied directly into the damaged site [
26], and constructs fabricated by 3D printing, with the desired shapes, have also been developed [
27]. Although these studies gave rise to interesting results in vitro, in animals and recently also in humans, currently no TE construct has been proposed that is able to regenerate all the anatomical structures of the nose [
28]. With the aim of further investigating in this field, several materials different from those tested so far could be tested. In this regard, polyvinyl alcohol (PVA) represents a valuable candidate due to its compatibility with many cells and tissues, non-toxicity and non-tumorigenicity, along with its hydrogel-forming capability, which leads to high water contents, similar to that of natural cartilage [
29].
In our recent studies, we showed that PVA-based sponges retain some key features of PVA hydrogels, being also able to widely modulate pore size to accomplish different cells and tissue structures [
30], thus becoming an interesting scaffold also in the otolaryngological field. By virtue of its hydrophilic nature, PVA can be easily blended with several biomolecules [
31]. PVA/gelatin (G) sponges, with highly interconnected pores, allow a good diffusion of nutrients and a high cell penetration. MSCs have demonstrated to differentiate into chondrocytes producing typical molecules of the cartilage extracellular matrix (ECM), both using traditional or scaffold-mediated culture methods [
32,
33]. These constructs subjected to mechanical stimulation in a bioreactor were also able to generate cartilage with elastic fibers, important for the reconstruction of the auricle [
34]. Finally, PVA/G sponges seeded with both bone marrow MSCs, differentiated into osteogenic lineage, and human umbilical vein endothelial cells (hUVECs), terminally differentiated towards endothelial lineage, showed first evidence of vascularized bone generation. This is important, in perspective, to improve implant viability during the regenerative process in vivo [
35]. MSCs can differentiate into all mesodermal tissues; however, an efficient differentiation into the endothelial phenotype is still a subject of investigation [
36]. The mesodermal (or mesangiogenic) progenitor cells (MPCs) are bone marrow precursors of the MSCs, which, being a step upstream in the mesengenic process, still have a strong potency for vasculogenesis [
37,
38]. Having a single cellular source to generate the most important structural tissues of the nose, including, but not limited to cartilage, bone, and vasculature, could definitively offer improved therapeutic solutions. Fibrocartilage is a tissue rich in type I collagen fibers, containing few chondrocytes; the fibers are organized in very dense bundles that make the fabric highly resistant to compression. It is a transition tissue between hyaline cartilage and dense connective tissue, typical of ligaments, also determinant for holding the nasal structures together and anchoring them to the face bones [
39].
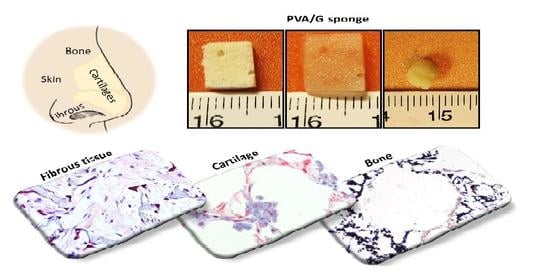
The aim of this study was to create a simple platform of three-dimensional (3D) cell/scaffold constructs for the regeneration of different nasal tissues by a single scaffold, the PVA/G sponge, and a single source of patient’s cells, the MPCs, able to generate different cell progenies: chondrogenic, osteogenic, endothelial and fibroblastic. We thus focused on the achievement of 3D constructs allowing the regeneration of hyaline cartilage, vascularized bone and fibrocartilage, which are the main connective tissues in the nose. Obtaining these constructs could represent a relevant step forward in repairing the variegated set of injuries affecting the different anatomical structures of the nose.
2. Materials and Methods
2.1. Materials
Polyvinyl alcohol (PVA; 99% hydrolyzed, Mw 85,000–146,000), Sodium Lauryl Sulphate (SLS), Phosphate buffered saline (PBS), Dulbecco’s Modified Eagle Medium (DMEM), Dulbecco’s Modified Eagle Medium Low Glucose (DMEM-LG), DMEM/F12, Insulin-transferrin-selenium (ITS), Pyruvate sodium, Dexamethasone, Trypsin, Nuclear fast red, Silver nitrate, Pyrogallole, Sodium Thiosulphate, Gelatin (G; type B from bovine skin), Glycine, Glutaraldehyde (GTA) grade II, L-Glutamine, Penicillin, Streptomycin, Xylene, Triton X-100, Bovine serum albumin (BSA), Mayer’s hematoxylin solution, Eosin, Dibutylphthalate Polystyrene Xylene (DPX) mounting medium, Methanol, Proteinase K, Pepstatin A, Iodoacetamide, Dimethylmethylene blue (DMMB), Ethylene Diamino Tetracetyc Acid (EDTA), Ampicillin, Fungizone, Gentamicin, b-glycerophosphate and MgCl2 were purchased from Sigma-Aldrich (Milan, Italy); Diflucan was purchased from Pfizer (Rome, Italy); Minimum Essential Medium, alpha modification was purchased from Cambrex/BioWhittaker (Walkersville, MD, USA); Human fibronectin and Matrigel® were purchased from BD Bioscience (Erembodegem, Belgium); Absolute ethanol and aluminum sulfate were bought from Carlo Erba (Milan, Italy); Citrate buffer was purchased from Diapath (Bologna, Italy); Ficoll Paque Plus, was purchased from GE healthcare (Hatfield, UK); StemMACS ChondroDiff Medium and Endocult were supplied by StemCell Technologies (Vancouver, Canada); Chromogen substrate 3-3′ diaminobenzidine tetrahydrochloride (DAB) was purchased from Amresco (Solon, OH, USA); Neutral buffered formalin and Alcian Blue kit, were purchased from Bio-Optica (Milan, Italy); Paraffin Histoplast LP and TrypLE Select were obtained from Thermo Fisher Scientific (Waltham, MA, USA); Transforming growth factor beta 1 (TGF-β1) was purchased from PeproTech (Rocky Hill, New Jersey); Vascular endothelial growth factor (VEGF) was purchased from Miltenyi Biotec (Cologne, Germany); AB human sera, Roswell Park Memorial Institute (RPMI-1640) medium and Osteogenic differentiation medium were purchased from Lonza (Basel, Switzerland); Heat-inactivated fetal bovine serum (FBS), Fetal Calf Serum (FCS) and Ascorbic acid were bought from Invitrogen (Carlsbad, CA, USA); PicoGreen kit was purchased from Molecular Probes (Eugene, OR, USA); Normal Goat Serum, Goat anti-mouse secondary antibody, Goat anti-rabbit secondary antibody and Vectastain Elite ABC Kit Standard were purchased from Vector Lab (Burlingame, CA, USA); mouse monoclonal anti-collagen type II (sc52658), rabbit polyclonal anti-Sox-9 (sc-20095), mouse monoclonal anti-Aggrecan (sc-33695), mouse monoclonal anti-Flk-1 (sc-6251), rabbit polyclonal anti-p-Flk-1 (sc-101820), mouse monoclonal anti-PECAM-1 (sc-81158), mouse monoclonal anti-Alkaline Phosphatase (ALP, sc-166261) and rabbit polyclonal anti-Osteocalcin (sc-30044) were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA, USA); rabbit polyclonal anti-collagen type I (ab34710) was purchased from AbCam (Cambridge, UK). LC Fast Start DNA Master SYBR Green kit and Tri Reagent®, LPS e LTA were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
2.2. Ethical Statement
Immortalized human keratinocytes, HaCaT cell line, were commercially obtained from ATCC-LGC Standards (Milan, Italy). Rat MSCs (rMSCs) were collected from discarded tissue from healthy rats sacrificed as controls in other studies approved by the local Ethical Committee. The experimental protocol followed the Principles of Laboratory Animal Care and specifically the re-use principle of the 3Rs. Human MPCs and human MSCs (hMSCs) were obtained by collecting the bone marrow overflowing from the medullary canal (i.e., discarded material) during prosthesis insertion in total hip replacement surgery of orthopedic patients. The samples were treated anonymously and in conformity to the principles expressed by the Declaration of Helsinki. As we used discarded anonymous material otherwise destinated to be disposed, ethical approval was not required.
2.3. Fabrication of PVA/G Sponges
Bioartificial PVA/G sponges were fabricated via emulsion and freeze-drying followed by chemical crosslinking. Specifically, an 11.72% aqueous solution of PVA prepared via autoclaving 1 h at 121 °C was cooled down to 50 °C under stirring at 1000 rpm in a glass flask placed inside a thermostatic bath. G was added to the PVA solution to reach a 20:80
w/
w and 30/70
w/
w ratio between the biomolecule G and the synthetic polymer PVA. A soft paste was obtained heating up the mixture to 71 °C for 10 min. Thereafter, the bath was cooled down again to 50 °C, 0.36% (
w/
v) of SLS was added to the mixture and stirring at 1000 rpm was continued for 10 min, leading to the obtainment of a voluminous dense foam that was spread on petri dishes with a large spatula, frozen at −80 °C and lyophilized. The dried foams were stabilized by crosslinking via GTA vapors for 72 h at 37 °C in a sealed cabinet and then flushed under the chemical hood for 48 h, thus obtaining biostable sponge-like materials. The crosslinking efficacy was tested by verifying the structural stability of the matrices in water for 7 days at 37 °C. The PVA/G sponge fabrication method is reported in detail in a previous study [
40].
2.4. Morphology and Surface Analysis of PVA/G Sponge
Scanning electron microscopy (SEM) was used to analyze the architectural features of the plain scaffolds. Samples were observed as obtained, post-dried (using either lyophilization or vacuum heating and after mechanical compression). The specimens were cross-sectioned, mounted on aluminum stubs, sputter-coated with gold (Edwards Sputter Coater S150B, Edwards, NY, USA) and examined under a scanning electron microscope JEOL JSM-5600 LV (Jeol Ltd., Tokyo, Japan) with an accelerating voltage of 12 kV.
The surface roughness analysis of lyophilized sponges was carried out with an atomic force microscope (AFM) (Molecular Imaging, TX, USA) equipped with silicon tips (nominal radii 10–20 nm) in air at 20 °C and 40% relative humidity. The analysis was performed working on tapping or contact mode and consisted of topographic data recording of square areas of approximately 40 × 40 μm2 (1024 × 1024 pixels). The roughness data were processed as the average among 15 different areas randomly selected upon the investigated surface.
2.5. Porosity and Pore Features of PVA/G Sponge Surfaces
Micro Computer Tomography (MicroCT) is a non-disruptive method recently used to measure 3D porosity and pore interconnectivity of scaffolds. A parallelepiped scaffold was scanned with a microCT imaging system (SkyScan 1172; SkyScan, Aartselaar, Belgium). Data were acquired and analyzed as previously reported [
37,
38]. Briefly, the raw micrographs were reconstructed using a Feldkamp algorithm. Then, grayscale thresholds of 45 (lower) and 255 (upper) were selected to have a realistic correspondence between grayscale tomograms and their binarized representations. Representative 3D reconstructions of the samples were generated to show a 3D model of the scaffold. Quantitative data were calculated considering a cylindrical volume of interest (VOI) within the boundaries of the scaffold. The total VOI selected in this study was 1.373 cm
3. Scaffold porosity was obtained using Equation (1):
in which V
bin obj is the binarized object volume within the total VOI. Thereafter, the scaffold interconnectivity was calculated as the void volumetric fraction accessible from the outside through pore openings of a predefined size [
37,
38]. In this study, a cut-off size of 4 voxels, equal to 51.2 μm, was defined as dimensionally compatible with MSC migration. The interconnected volume was estimated via a “shrink-wrap” process, as reported in the CT Analyzer instructions. The percentage of inaccessible volume can be finally determined in Equation (2), as:
Here, the terms POST and PRE refer to after and before the shrink-wrap process, respectively. Finally, interconnectivity percent can be calculated as the complement to the inaccessible volume percent.
2.6. Water Content and Mechanical Characterization of PVA/G Sponges
A lyophilized sponge was puncher-cut into cylinders (
n = 6) with diameter (D) = 8 mm and height (H) ranging in 4.2–4.8 mm, as measured with an electronic caliper (Mitutoyo, Tokyo, Japan). The specimens were equilibrated in PBS (PBS; Sigma-Aldrich) 0.1 M, pH 7.4, for 2 h at 37 °C. Excess of liquid was carefully removed leading to the evaluation of their wet weight (V
wet). The wet sponges were placed under a laminar flow hood for 3 days. the volume increase percent were calculated with respect to the wet and dry conditions, using Equation (3), in which
wet refers to either wet or post-dry volume condition.
A bench dynamometer (INSTRON 5542, 50 N load cell, UK) was used to test samples in unconfined compression between two 50 mm diameter non-porous stainless-steel platens. Six samples, previously subjected to the above-described water content analysis, underwent a compressive ramp up to 50% strain at a strain rate of 0.0167 mm/s. Some drops of double-distilled water were placed around the samples to ensure that they remained hydrated throughout testing. The tangent slope was measured by calculating a baseline estimate for localized data at 10% increments from 10% to 50% strain.
2.7. Isolation and Expansion of Bone Marrow Stem Cells
This study availed itself of bone marrow-derived stem cells of rat and human origin. Mononuclear cells were collected from human bone marrow aspirates by density gradient centrifugation on Ficoll-Paque Plus, and plated in DMEM-LG supplemented with 10% of pooled human AB sera at 0.8 × 10
6/cm
2 on hydrophobic untreated flasks. After 48–72 h, nonadherent cells were discarded and fresh medium was added. After 8–10 days, the cells were detached with TrypLE Select for 15–20 min at 37 °C, in 5% CO
2 cell incubator obtaining MPCs, a bone marrow stem cell population resting and more immature than MSCs, able to differentiate towards the mesenchymal and endothelial lineages. HMSCs were thus obtained from human MPCs by replacing human AB serum with FBS, as reported in previous studies [
41]. HMSCs cultured using DMEM-LG supplemented with 10% FBS, 100 IU/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, 100× Diflucan (2 mg/mL). Cells were seeded at a density of 200,000 cells/cm
2 and cultured in in 5% CO
2 cell incubator. After 48–72 h nonadherent cells were discarded and fresh medium was added. When 80% confluence was reached adherent cells were trypsinized and seeded at low density for further expansion.
Finally, rMSCs were harvested from the tibiae and femora of 41–44-days-old male Wistar rats (Charles River Laboratories, USA) using established method as previously described [
42]. Briefly, the leg bones were excided, the soft tissue was removed, and the leg bones were placed in DMEM supplemented with 200 µg/mL of PSF cocktail. This concentration of antibiotics is 4 times superior to the normal concentration used in cell culture to avoid contamination during the harvest process. The proximal end of the femur and distal end of the tibia were clipped. An 18-gauge needle was inserted into the hole in knee joint in each bone, and the bone was flushed with 5 mL of complete osteogenic medium (α-MEM supplemented with 10% FBS, 50 µg/mL gentamicin, 100 µg/mL ampicillin, 10 mM fungizone, 50 µg/mL L-ascorbic acid, 0.01 M β-glycerophosphate, and 0.01 M dexamethasone). The resulting marrow pellet was broken up by trituration, and cell suspensions from all bones were combined in a centrifuge tube. The cells were plated in 75 cm
2 flasks and cultured for 6 days in complete osteogenic media at 37 °C in a humidified atmosphere of 95% air, 5% CO
2 to allow the expansion of the cells. Medium was changed at 1 and 3 days to remove the non-adherent cell population.
2.8. PVA/G Scaffold Preparation and Generation of 3D Models
The sponges, 80/20 and 70/30 (w/w%) PVA/G, were puncher cut into cylinders (5 mm diameter, 3 mm or 1.5 mm thickness) that were sterilized with absolute ethanol overnight, washed three times, 10 min each, with sterile 3× Pen-Strep/Diflucan in saline, rinsed in sterile PBS and finally treated with a sterile-filtered 2 M aqueous solution of glycine for 1 h to block GTA unreacted binding sites. Thereafter, the scaffolds were washed with sterile PBS to remove excess glycine prior to cell seeding.
2.8.1. Chondrogenic Differentiation of MSCs in PVA/G Scaffolds
RMSCs at passage 2 were seeded on 80/20 (w/w%) PVA/G scaffolds (3 mm thickness) at a density of 500,000 cells/sample using regular culture medium for adhesion. After 24 h, the cell/scaffold constructs were committed to the chondrogenic lineage, replacing the regular culture medium with differentiating culture medium, consisting of DMEM:F12, 1.25 μg/mL bovine serum albumin, 5.35 μg/mL linoleic acid, 50 μg/mL ascorbic acid, 100 μg/mL sodium pyruvate, ITS, 10−7 M dexamethasone and 10 ng/mL and TGF-β1. Differentiation was carried out for 10 days and 20 days, providing culture medium changes every 3 days.
2.8.2. Vasculogenic and Osteogenic Differentiation of MPCs in PVA/G Sponges
To perform pre-endothelial differentiation, MPCs were plated at 10,000 cells/cm2 in Endocult differentiation medium on 25 cm2 tissue culture flasks coated with human fibronectin and cultured for 2–3 weeks. Pre-endothelial cells were then detached with trypsin digestion and counted to seeding them on scaffolds. Parallel cultures were performed on chamber slides previously coated with Matrigel® (100 µL/well) to obtain terminal differentiation. After 18 h, chamber slides were fixed in 1% w/v neutral buffered formalin for 10 min at 4 °C. In the meantime, the 80/20 (w/w%) PVA/G scaffolds (1.5 mm thickness) were seeded with hMSCs at the density of 500,000 cell/scaffold and the constructs were osteodifferentiated for 14 days using OsteoDiff Medium. Osteoblast/PVA constructs were then coated with Matrigel (100 µL/scaffold) and MPC-derived pre-endothelial cells obtained from Endocult cultures were seeded at the density of 400,000 cell/scaffold. Terminal endothelial differentiation was performed by incubating the constructs in Endocult medium containing 50 ng/mL of VEGF in cell culture incubator for 18 h.
2.8.3. Fibrocartilaginous Differentiation of MSCs in PVA/G Scaffolds
HMSCs at passage 2 were trypsinized and seeded in 75 cm2 flasks at a density of 5000 cells/cm. After 4 days of expansion in DMEM, cells were committed to chondrogenic lineage by adding StemMACS ChondroDiff Medium and pre-differentiated for 4 days. After the pre-differentiation in 2D conditions, the chondroprogenitor cells were trypsinized, seeded on 70/30 (w/w%) PVA/G scaffolds (1.5 mm thickness) at a density of 300,000 cells/sample and differentiated for additional 24 days.
2.9. 3D Model Characterization
2.9.1. Histological Characterization of 3D Models
After formalin fixation, constructs employed to the creation of chondrogenic, vascularized osteogenic and fibrocartilaginous models were dehydrated with a graded series of ethanol (from 70% to 100%). After 3 h of incubation in absolute ethanol (Bio-Optica), samples were clarified in xylene (two steps of 45 min each) (Sigma-Aldrich). All steps were performed in a thermostatic bath at 40 °C. Successively, specimens were rinsed in liquid paraffin (Histoplast LP, Thermo Fisher Scientific, Waltham, MA, USA) at 60 °C for 2 h and paraffin-embedded. Sections 5 µm thick were obtained by standard microtome and mounted onto glass slides. Before each histological staining or immunoreaction, sections were deparaffined in xylene (two steps of 7 min each), rehydrated in absolute ethanol (three steps of 7 min each) and rinsed in distilled water for 5 min. Before each staining, sections of both seeded PVA and chondrogenic pellet were deparaffinized; after each staining, the sections were dehydrated.
Sections were deparaffinized rinsing them in xylene twice for 7 min, then they were rehydrated by absolute ethanol three times for 7 min and washing them in distilled water for 5 min. Samples were then stained with Mayer’s hematoxylin for 5 min, washed in tap water for 5 min and counterstained with 1% w/v eosin for 1 min. After washing in distilled water, specimens were dehydrated by three washes in absolute ethanol and clarified by three washes in xylene (5 min for each step), then they were mounted with a coverslip by DPX mounting agent.
Specimens were incubated in Alcian Blue pH 1 solutions for 30 min and in revealing solutions for 10 min, according to manufacturer’s instructions. After the incubation time, sections were washed in distilled water, counterstained with a distilled water solution containing 0.1% w/v Nuclear Fast Red and 5% w/v aluminum sulfate for 5 min and washed in tap water for 5 min. Sections were dehydrated and mounted as previously described.
Deparaffinized sections were incubated with 1% w/v silver nitrate exposed to light for 15 min, 0.5% w/v Pyrogallol for 2 min and 5% w/v sodium thiosulphate for 2 min. All the solutions were made in distilled water. After each passage, washings in distilled water were performed. The counterstaining was performed by incubating cells with 0.1% w/v nuclear fast red diluted in a distilled water solution containing 5% w/v aluminum sulphate, for 5 min and washing in tap water for 5 min in order to reveal the reaction. Sections were dehydrated and mounted as previously described.
Sections employed for the detection of aggrecan and collagen type II were incubated in citrate buffer in a thermostatic bath at 90 °C for 30 min, in order to unmask antigens, then they were cooled down for 30 min and washed in 1× PBS for 10 min; cells and sections employed for the detection of the other antigens were permeabilized with 0.2% w/v Triton X-100 in 1× PBS for 10 min. All specimens were incubated in a methanolic solution containing 0.06% v/v H₂O₂ in the dark for 15 min to quench peroxidases. Sections were rinsed in distilled water and washed in 1× PBS. Therefore, specimens were incubated with 5% v/v goat serum diluted in 1x PBS for 20 min at 37 °C to block aspecific binding sites of the secondary antibodies. Specimens were washed in 1x PBS and then incubated with primary antibodies diluted in 0.1% w/v BSA/1x PBS solution in a moist chamber overnight at 4 °C. The following antibodies with specified concentrations were used: anti-aggrecan 1:50; anti-Sox-9 1:100; anti-collagen type II 1:50, anti-collagen type I 1:1000, anti-ALP 1:100, anti-Osteocalcin 1:400, anti-TGFβ-1 1:100, anti-Flk-1 1:50, anti-p-Flk-1 1:50, anti-PECAM-1 1:100. Negative controls were obtained by incubating some sections with 0.1% w/v BSA/1x PBS only. The next day, specimens were incubated with goat anti-rabbit or goat anti-mouse biotinylated secondary antibodies diluted 1:200 in 1.5% v/v goat serum-1x PBS solution for 60 min, then streptavidin solution was added for 30 min, prepared according to manufacturer’s instructions reported in the Vectastain Elite ABC Kit Standard. To reveal the reaction, the sections were incubated in the substrate-chromogen solution (0.5 mg/mL 3,3′-diaminobenzidine tetrahydrochloride activated by H₂O₂) for 5 min in the dark, then they were counterstained with Mayer’s hematoxylin for 2 min and washed in tap water for 2 min. Finally, the sections were dehydrated, clarified and mounted in DPX medium. In the second part of the reaction, after each passage, washings in 0.01% v/v Triton/PBS 1× and 1× PBS solutions were performed. All the histological analyses were observed with a Nikon Eclipse Ci microscope (Nikon Instruments, Amsterdam, The Netherlands) and the images were acquired by a digital camera equipped on the microscope.
2.9.2. Morphological Analysis of Chondrogenic 3D Model
After fixation and washing in 1×PBS, constructs employed to the creation of the chondrogenic model were dehydrated with a graded series of ethanol ethanol/water solutions up to anhydrous ethanol. The specimens were dried using the critical point method (Balzers CPD030, Oerlikon Balzers, Balzers, Liechtenstein), then mounted on aluminum stumps, sputter-coated with gold and examined using a scanning electron microscope as previously reported.
To evaluate the tendence of rMSCs to form groups in the chondrogenic model, rounded total cells (RTC) and rounded cell groups (RCG) were counted in nine histological sections; sections were collected one each 10–15 µm from sectioned constructs.
At the selected time-points of the chondrogenic model (10 and 20 culture days), the culture medium was removed, and the samples were stored at −80 °C for quantitative assays using polypropylene tubes containing a sterile enzymatic solution. The digestive solution consisted of proteinase K, pepstatin A and iodoacetamide dissolved in EDTA. Cell lysates were obtained using freeze-thaw cycles each, namely, quench in liquid N
2, defrosting at 37 °C followed by vortexing. Each cycle had the duration of 30 min and was repeated three times. Following this procedure, the contents of double stranded (ds)-DNA and GAGs could be thus quantified in cascade on the same specimens (
n = 3). Ds-DNA content in cellular lysates was measured using the PicoGreen assay, as previously reported [
43]. Briefly, working buffer and PicoGreen dye solution were prepared according to the manufacturer’s instructions using reagents provided within the kit. After 10 min of incubation in the dark at room temperature, the fluorescence intensity of the samples was measured on a plate reader (Victor3; PerkinElmer, Waltham, MA, USA), using an excitation wavelength of 485 nm and an emission wavelength of 535 nm. To determine the GAG content, the samples immersed in their residual lysates were processed for the enzymatic digestion, which was carried out inside a water bath for 16 h at 60 °C. Thereafter, the DMMB assay was performed, as previously reported [
43]. Absorbance was measured at 520 nm using a plate spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA). Results were expressed as the ratio between GAG content and ds-DNA content at 10 and 20 culture days. Values were then analyzed by statistical analysis.
2.10. Epithelial Cytokine Evaluation
The immunomodulatory properties of the scaffolds (1.5 mm thickness) were studied on skin keratinocytes HaCaT via Real Time PCR, using the primers and parameters reported in
Table 1.
Immortalized human keratinocyte HaCaT cell line was expanded in DMEM-HG supplemented with 1% Penstrep, 1% glutamine and 10% fetal calf serum at 37 °C in air and 5% CO
2. When 80% confluence was reached, 2 × 10
6 cells resuspended in 50 µL of DMEM were seeded on sponges of 70/30 (
w/w%) PVA/G placed in 96-well plates. After 4 h from seeding, 100 µL of DMEM were added to the wells and incubated for 24 h. The same number of cells was seeded in a control well without the sponges. To verify the anti-inflammatory activity of the tested sponges, some wells were treated with lipopolysaccharide (LPS) of
Pseudomonas aeruginosa and lipoteichoic acid (LTA) of
Staphylococcus aureus (both at concentration of 10 µg/mL), for additional 24 h. A cell seeded well, in which no inflammatory molecule was added, was intended as untreated control. At the endpoint, total RNA was isolated with Tri-Reagent and 1 µg was reverse-transcribed into complementary DNA (cDNA) using random hexamer primers, at 42 °C for 45 min, according to the manufacturer’s instructions. Real time PCR was carried out with the LC Fast Start DNA Master SYBR Green kit using 2 µL of cDNA, corresponding to 10 ng of total RNA in a 20 µL final volume, 3 mM MgCl
2 and 0.5 µM sense and antisense primers. Real-Time PCR were carried out in order to evaluate the expression of IL-1α, IL-1β, TNF-α, IL-6 and IL-8, TGF-β and hBD-2 (
Table 1).
2.11. Statistical Analysis
Statistical analysis was carried out using Student’s t-test. The analysis was conducted by comparing the data of independent experiments and the differences were considered statistically significant for p < 0.05. Data are expressed as mean ± standard deviation.
4. Discussion
The nose is a complex organ consisting of several tissue types that are mechanically and functionally interconnected with each other to prime the respiratory function: cartilaginous, bony, epithelial, adipose and fibromuscular tissues, among others. The shape and integrity of all the nasal tissues are fundamental for its physiology. Due to its exposed position, the nose is affected by several pathologies, mainly derived from allergy, infections, traumas, burns, chemical agents, drugs, and tumors [
1,
2,
3,
4]. In particular, tumor resection leads to deformation of the nasal structures with impairment of its function and aesthetics, thus necessitating reconstructive surgery, which is expensive and complex, as it requires several interventions. Due to its specific function and location, and for environmental factors like air pollution, the nose is challenged by inflammatory processes. Therefore, novel biomaterial approaches able to provide safe repair and reconstruction are greatly needed. Having a biomaterial suitable for reconstructing different nasal tissues would offer a better versatile platform for rhinoplasty.
Recently, the problem of nasal tissue regeneration has been addressed from a TE point of view. In recent decades, the TE paradigm, comprising cells, 3D biomaterial scaffolds and appropriate stimuli, has been explored in many medical fields, including nose reconstruction, albeit in limited studies. Although good results have been obtained, recently also in humans, no TE construct has yet been found to regenerate damaged nose in all its components [
28].
Starting from these assumptions, we generated in vitro constructs made of PVA/G scaffolds and stem cells differentiated towards different lineages and we performed a morpho-functional investigation to assess potential to obtain cartilage tissue for repairing septum and lateral cartilages, bone tissue for the vomer and other bone structures, fibrous tissue for the regeneration of the connective ligaments and alae. PVA is a very versatile and widely known biocompatible material, used in many TE studies, but not yet evaluated for nasal structures [
44]. G is a natural protein obtained from collagen denaturation. As the denaturation process preserves many functional groups of native collagen, G is able to interact with cells and facilitate their adhesion [
45]. Moreover, we demonstrated that the addition of G during the emulsion process helps the foam porogenesis, which ultimately leads to pore size distribution in the range of cellular size [
35]. Overall, G affects pore formation, size and interconnectivity via foaming, is responsible for sponge stability via GTA crosslinking, and modulates the surface properties of PVA to trigger cell adhesion. Considering our previous studies on PVA/G sponges, we observed that G within 20–30% by weight with respect to PVA led to sponges suitable for the growth of several cell types [
30,
34,
35,
40]. In fact, as we showed here, both 80/20 and 70/30 (w/w%) PVA/G sponges displayed a highly porous architecture, with round-shaped pores and quite smooth surfaces. The sponges demonstrated a volumetric porosity of ~ 85% and pore interconnectivity of ~97%, measured by considering minimum pore-pore openings of 52 µm. This evidence indicates that only ~3% of the scaffold volume is either inaccessible or connected through lower size openings, which, however, can contribute to oxygen and nutrient diffusion across the scaffold. Upon wetting, both sponge types almost doubled their volume, ultimately reaching a water content similar to that of cartilage [
29]. Wet sponges were extremely soft in nature, as corroborated by compressive mechanical tests, and were provided with shape recovery, which is a remarkable feature for minimally invasive surgery. Both formulations thus showed very similar behavior, which was considered to fit nasal applications.
In addition to providing a scaffold suitable for diverse nasal tissues, the second key point of this study was to suggest a unique cell source for regenerating those tissues, and in particular, cartilage, pre-vascularized bone and fibrous tissue. We thus proposed the use of MPCs isolated from the human bone marrow, as they are able to give rise to pre-endothelial cells and to MSCs, the latter capable of differentiating into several mesodermal lineages [
37,
38,
41]. In a previous work, we tested PVA/G scaffolds for the regeneration auricle cartilage, finding that it was promising for elastic cartilage engineering [
34]. In fact, we obtained elastic fibers by applying mechanical forces during the chondrogenic differentiation of MSCs. In this study, rMSCs seeded on the PVA/G sponges were differentiated towards the chondrogenic phenotype aimed to obtain a hyaline cartilage model. MSCs proliferated and chondro-differentiated efficiently in PVA/G scaffolds. The obtained results showed that the cells colonized the scaffold also in depth. Moreover, differently from other cell sources, like dental pulp [
43], the bone marrow MSCs in this scaffold massively assumed a rounded morphology and acquired the ability to aggregate into clusters within its pores already after 10 days, but mainly after 20 days of chondrogenic differentiation. Differentiated MSCs also gave rise to the production of molecules typical of hyaline cartilage, such as sulphated acidic GAGs and collagen type II. As aggregation into isogenic groups is a hallmark of hyaline cartilage, the positivity to these specific chondrogenic markers indicates that this scaffold is advantageous to support chondrogenic differentiation of bone marrow MSCs [
46]. We thereafter used hMSCs to generate a panel of 3D nasal tissue models. Unlike cartilage, bone is vascularized tissue. The key steps towards optimized clinical applications of tissue-engineered bone substitutes rely on neovascularization of the engineered construct after implantation [
47]. For this reason, strategies enabling subsequent in vivo vasculogenesis, such as the addition of vascular endothelial cells are being investigated [
35]. In the pre-vascularized osteogenic model, we aimed to exploit the angiogenetic and osteogenic potential of a specific subpopulation of bone marrow cells, recently known as MPCs. In parallel cultures, MPCs were differentiated either into pre-endothelial cells or into MSCs, the latter further seeded on the scaffolds upon osteogenic differentiation. The pre-endothelial cells differentiated in 2D culture were then seeded on the 3D bone model generated in the meantime for a final coculture step, as similarly reported in a previous study [
35]. In the obtained construct, the production of bone ECM and the expression of osteogenic markers, such as ALP and OCN, along with the strong immunopositivity for the VEGF receptor Flk-1 highlighted in some cells, suggested that the PVA/G scaffold allowed both osteogenic and endothelial differentiation of MPCs. This evidence is confirmed by the comparison with MPCs differentiated in 2D culture, in which this protein was poorly expressed. On the other hand, PECAM and the phosphorylated form of the Flk-1 receptor, weakly expressed in the 2D culture, were absent in our 3D model, indicating the need to further investigate the role of the PVA/G sponge in the endothelial differentiation of MPCs, due to their early angiogenic potential [
38]. This pre-vascularized bone model retraced the findings previously obtained using hUVECs and hMSCs by instead using a single cell source, patient-derived, which better opens up translational perspectives [
35].
PVA/G sponges are mechanically soft materials, although the addition of G is able to increase the sponge elastic modulus by virtue of the chemical crosslinking [
34,
48]. In general, for PVA scaffolds, a low mechanical strength has been reported, which may not be suitable for skeletal bone engineering [
49]. Conversely, as a part of the facial bones, the nasal bone is not subjected to mechanical loading, so other characteristics, such as high porosity, large pore size and water absorption capacity could play a relevant role in this context. Most importantly, a material that allows tissue continuity between the different structural parts of the nose is needed. In fact, the bone ECM secreted by the cells could finally provide the necessary mechanical strength proper of bone. The hMSCs obtained from the MPCs were finally used to generate a 3D model of fibrocartilage. By tuning the differentiative stimuli in the culture, we selected an intermediate cell population producing massive amount of collagen type I but still retaining some chondrogenic characteristics, as shown by the production of sulphated acidic GAGs and by the immunopositivity for the transcription factor Sox-9. Our fibrocartilage tissue model is representative of the connective tissue that builds up the ligaments connecting the nose anatomical structures, the alar tissue, and that anchors the nose to the facial bones [
39]. All in all, we showed that PVA/G sponges and a single cell source, i.e., the MPCs, have a wide potential for the regeneration of nasal tissues, which could be tuned by varying the culture conditions (i.e., biochemical stimuli). Possessing several features on different length scales, such as properly distributed pore sizes showing surfaces of suitable roughness [
50,
51], along with befitting chemical moieties provided by a natural biopolymer in proper concentration, PVA/G scaffolds allowed the regeneration of several mesodermal tissues, including zonal transition tissue, which are greatly important in TE applications [
52].
As a first defense line for our breathing organs, the nose is naturally exposed to pathogens, including viruses, fungi and bacteria, which interact with the nasal epithelium. To respond pathogenic insults, our epithelia can secrete pro-inflammatory cytokines that trigger immune response, as well as antimicrobial peptides as for innate immunity [
53]. A recurrently inflamed or infected microenvironment challenges the presence of biomaterials implanted in the human body, leading to extrusion phenomena when synthetic non-bioresorbable materials are used. At the end of the nose surgery, de-epithelialized areas of the nasal septum or fossa need to be protected to avoid bacterial infiltration and biofilm formation [
54]. We thus explored whether PVA/G scaffolds could interfere with the immunomodulatory response performed by epithelial cells. Immortalized human keratinocyte, HaCaT cells, were put in contact with PVA/G sponges and incubated with two types of molecules produced by bacteria, and thus were able to promote inflammatory processes led by epithelial cells: LPS from
Pseudomonas aeruginosa and LTA from
Staphylococcus aureus. Gene expression of the pro-inflammatory cytokines involved in the immune response was evaluated by comparing the HaCaT cells growth in contact with PVA/G scaffolds and alone. Our findings showed a similar behavior between these two culture conditions, thus suggesting that PVA/G scaffolds should not significantly affect the epithelial inflammatory response toward microbial agents. Moreover, the presence of G in combination with synthetic polymers can enhance the overall biocompatibility, as also demonstrated by previous studies [
55,
56].
Having a simple scaffold/stem cell platform for the regeneration of several nasal tissues would promote better satisfactory esthetic and functional outcomes in rhinoplasty, including reconstruction after sinonasal tumor surgery.