Comprehensive Study of Variety Oenological Potential Using Statistic Tools for the Efficient Use of Non-Renewable Resources
Abstract
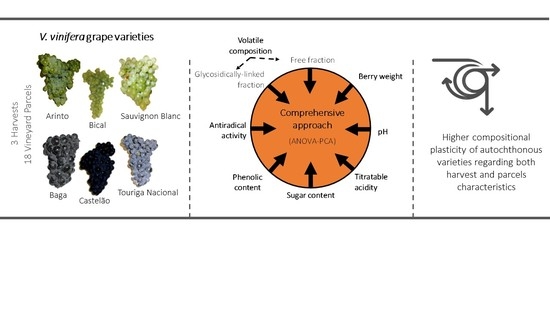
:1. Introduction
2. Materials and Methods
2.1. Samples, Vineyards and Harvests Characteristics, and Sampling
2.1.1. Samples
2.1.2. Vineyard Parcels Characteristics
2.1.3. Harvest Weather Conditions
2.1.4. Sampling
2.2. Grapes Physicochemical Parameters Determination
2.2.1. Classical Physicochemical Parameters
2.2.2. Total Phenolic Content
2.2.3. Antiradical Activity
2.3. Grapes Volatile Composition Determination
2.3.1. Determination of Grapes Free Volatile Profiles–HS-SPME Procedure
2.3.2. Determination of Grapes’ Glycosidically Linked Profiles—SPE Procedure
2.3.3. GC×GC-ToFMS Analysis
2.4. Data Processing
3. Results and Discussion
3.1. Grapes Physicochemical Composition Evaluation
3.2. Grapes’ Volatile Profile Evaluation
3.3. Statistic Tools to Evaluate Each Variety Oenological Potential
- Arinto
- Bical
- Sauvignon Blanc
- Baga
- Castelão
- Touriga Nacional
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Petronilho, S.; Barros, A.S.; Coimbra, M.A.; Rocha, S.M. Efficient Use of Non-renewable Natural Resources for Quality Wine through Sustainable Viticulture. In Agricultural Systems in the 21st Century; Nova Publishers: Hauppauge, NY, USA, 2013; pp. 195–230. [Google Scholar]
- Lamastra, L.; Fragkoulis, G.; Trevisan, M.; Capri, E. Enhancing the Ecosystem Services in Viticulture Farms: Approaches towards a Sustainable Management. In Environmental Management; Sarkar, S., Ed.; Sciyo InTech: Rijeka, Croatia, 2010; pp. 69–94. [Google Scholar]
- Coelho, E.; Coimbra, M.A.; Nogueira, J.M.F.; Rocha, S.M. Quantification approach for assessment of sparkling wine volatiles from different soils, ripening stages, and varieties by stir bar sorptive extraction with liquid desorption. Anal. Chim. Acta 2009, 635, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Suklje, K.; Carlin, S.; Antalick, G.; Blackman, J.; Deloire, A.; Vrhovsek, U.; Schmidtke, L. Regional Discrimination of Australian Shiraz Wine Volatome by Two-Dimensional Gas Chromatography Coupled to Time-of-Flight Mass Spectrometry. J. Agricult. Food Chem. 2019, 67, 10273–10284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.K.; Kontoudakis, N.; Šuklje, K.; Antalick, G.; Blackman, J.W.; Rutledge, D.N.; Schmidtke, L.M.; Clark, A.C. Changes in Red Wine Composition during Bottle Aging: Impacts of Grape Variety, Vineyard Location, Maturity, and Oxygen Availability during Aging. J. Agric. Food Chem. 2020, 68, 13331–13343. [Google Scholar] [CrossRef]
- Anesi, A.; Stocchero, M.; Dal Santo, S.; Commisso, M.; Zenoni, S.; Ceoldo, S.; Tornielli, G.; Siebert, T.; Herderich, M.; Pezzotti, M.; et al. Towards a scientific interpretation of the terroir concept: Plasticity of the grape berry metabolome. BMC Plant Biol. 2015, 15, 191–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, G.V.; Davis, R. Climate influences on grapevine phenology, grape composition, and wine production and quality for Bordeaux, France. Am. J. Enol. Vitic. 2000, 51, 249–261. [Google Scholar]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate change and global wine quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Jackson, D.I.; Lombard, P.B. Environmental and management practices affecting grape composition and wine quality—Rewiew. Am. J. Enol. Vitic. 1993, 44, 409–430. [Google Scholar]
- Martinez, R.F.; Ascacibar, F.J.M.-d.-P.; Espinoza, A.V.P.; Lorza, R.L. Predictive modelling in grape berry weight during maturation process: Comparison of data mining, statistical and artificial intelligence techniques. Span. J. Agric. Res. 2011, 9, 1156–1167. [Google Scholar] [CrossRef]
- Marais, J.; Calitz, F.; Haasbroek, P.D. Relationship between microclimatic data, aroma component concentrations and wine quality parameters in the prediction of Sauvignon blanc wine quality. S. Afr. J. Enol. Vitic. 2001, 22, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.O.; Wample, R.L.; Sachidhanantham, S.; Kaye, O. Grape Quality Mapping for Vineyard Differential Harvesting. Braz. Arch. Biol. Technol. 2012, 55, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.A.; Malheiro, A.C.; Karremann, M.K.; Pinto, J.G. Statistical modelling of grapevine yield in the Port Wine region under present and future climate conditions. Int. J. Biometeorol. 2011, 55, 119–131. [Google Scholar] [CrossRef]
- Niimi, J.; Tomic, O.; Naes, T.; Bastian, S.; Jeffery, D.; Nicholson, E.; Maffei, S.; Boss, P. Objective measures of grape quality: From Cabernet Sauvignon grape composition to wine sensory characteristics. Lwt Food Sci. Technol. 2020, 123, 109105–109114. [Google Scholar] [CrossRef]
- Godelmann, R.; Fang, F.; Humpfer, E.; Schutz, B.; Bansbach, M.; Schafer, H.; Spraul, M. Targeted and Nontargeted Wine Analysis by H-1 NMR Spectroscopy Combined with Multivariate Statistical Analysis. Differentiation of Important Parameters: Grape Variety, Geographical Origin, Year of Vintage. J. Agric. Food Chem. 2013, 61, 5610–5619. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, D.; Sun, D.; Gorecki, A.; Blaszczak, W.; Fornal, J.; Jelinski, T. Quantitative and predictive study of the evolution of wine quality parameters during high hydrostatic pressure processing. Innov. Food Sci. Emerg. 2013, 20, 81–90. [Google Scholar] [CrossRef]
- Rudnitskaya, A.; Rocha, S.; Legin, A.; Pereira, V.; Marques, J. Evaluation of the feasibility of the electronic tongue as a rapid analytical tool for wine age prediction and quantification of the organic acids and phenolic compounds. The case-study of Madeira wine. Anal. Chim. Acta 2010, 662, 82–89. [Google Scholar] [CrossRef]
- Firmani, P.; Vitale, R.; Ruckebusch, C.; Marini, F. ANOVA-Simultaneous Component analysis modelling of low-level-fused spectroscopic data: A food chemistry case-study. Anal. Chim. Acta 2020, 1125, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Marcheafave, G.G.; Tormena, C.D.; Mattos, L.E.; Liberatti, V.R.; Ferrari, A.B.S.; Rakocevic, M.; Bruns, R.E.; Scarminio, I.S.; Pauli, E.D. The main effects of elevated CO2 and soil-water deficiency on 1H NMR-based metabolic fingerprints of Coffea arabica beans by factorial and mixture design. Sci. Total Environ. 2020, 749, 142350–142360. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Hoefsloot, H.; van der Greef, J.; Timmerman, M.; Westerhuis, J.; Smilde, A. ASCA: Analysis of multivariate data obtained from an experimental design. J. Chemom. 2005, 19, 469–481. [Google Scholar] [CrossRef]
- Smilde, A.; Jansen, J.; Hoefsloot, H.; Lamers, R.; van der Greef, J.; Timmerman, M. ANOVA-simultaneous component analysis (ASCA): A new tool for analyzing designed metabolomics data. Bioinformatics 2005, 21, 3043–3048. [Google Scholar] [CrossRef] [PubMed]
- Meyners, M. Permutation tests: Are there differences in product liking? Food Qual. Pref. 2001, 12, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Vis, D.; Westerhuis, J.; Smilde, A.; van der Greef, J. Statistical validation of megavariate effects in ASCA. BMC Bioinform. 2007, 8, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westerhuis, J.; Hoefsloot, H.; Smit, S.; Vis, D.; Smilde, A.; van Velzen, E.; van Duijnhoven, J.; van Dorsten, F. Assessment of PLSDA cross validation. Metabolomics 2008, 4, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Coelho, E.; Rocha, S.; Delgadillo, I.; Coimbra, M. Headspace-SPME applied to varietal volatile components evolution during Vitis vinifera L. cv. ‘Baga’ ripening. Anal. Chim. Acta 2006, 563, 204–214. [Google Scholar] [CrossRef]
- Perestrelo, R.; Petronilho, S.; Câmara, J.S.; Rocha, S.M. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry combined with solid phase microextraction as a powerful tool for quantification of ethyl carbamate in fortified wines. The case study of Madeira wine. J. Chromatogr. A 2010, 1217, 3441–3445. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Coutinho, P.; Barros, A.; Coimbra, M.A.; Delgadillo, I.; Cardoso, A.D. Aroma Potential of Two Bairrada White Grape Varieties: Maria Gomes and Bical. J. Agric. Food Chem. 2000, 48, 4802–4807. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.P.; Gonçalves Silva, D.; Rudnitskaya, A.; Almeida, A.; Rocha, S.M. Shedding light on Aspergillus niger volatile exometabolome. Sci. Rep. 2016, 6, 27441–27454. [Google Scholar] [CrossRef]
- Rocha, S.M.; Freitas, R.; Cardoso, P.; Santos, M.; Martins, R.; Figueira, E. Exploring the potentialities of comprehensive two-dimensional gas chromatography coupled to time of flight mass spectrometry to distinguish bivalve species: Comparison of two clam species (Venerupis decussata and Venerupis philippinarum). J. Chromatogr. A 2013, 1315, 152–161. [Google Scholar] [CrossRef]
- Mondello, L. HS-SPME-GCxGC-MS analysis of Yerba Mate (Ilex paraguariensis) in Shimadzu GC-GC application compendium of comprehensive 2D GC. Shimadzu Corp. 2012, 1, 1–29. [Google Scholar]
- Robinson, A.L.; Boss, P.K.; Heymann, H.; Solomon, P.S.; Trengove, R.D. Development of a sensitive non-targeted method for characterizing the wine volatile profile using HS-SPME/GC×GC-TOFMS. J. Chromatogr. A 2011, 1218, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.M.; Coelho, E.; Zrostlíková, J.; Delgadillo, I.; Coimbra, M.A. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry of monoterpenoids as a powerful tool for grape origin traceability. J. Chromatogr. A 2007, 1161, 292–299. [Google Scholar] [CrossRef]
- Jalali, H.T.; Petronilho, S.; Villaverde, J.J.; Coimbra, M.A.; Domingues, M.R.M.; Ebrahimian, Z.J.; Silvestre, A.J.D.; Rocha, S.M. Deeper insight into the monoterpenic composition of Ferula gummosa oleo-gum-resin from Iran. Ind. Crops Prod. 2012, 36, 500–507. [Google Scholar] [CrossRef]
- Jalali-Heravi, M.; Zekavat, B.; Sereshti, H. Characterization of essential oil components of Iranian geranium oil using gas chromatography-mass spectrometry combined with chemometric resolution techniques. J. Chromatogr. A 2006, 1114, 154–163. [Google Scholar] [CrossRef]
- Kim, M.R.; Abd El-Aty, A.M.; Kim, I.S.; Shim, J.H. Determination of volatile flavor components in danggui cultivars by solvent free injection and hydrodistillation followed by gas chromatographic-mass spectrometric analysis. J. Chromatogr. A 2006, 1116, 259–264. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Morelli, I. Differences in the fragrances of pollen and different floral parts of male and female flowers of Laurus nobilis. J. Agric. Food Chem. 2002, 50, 4647–4652. [Google Scholar] [CrossRef] [PubMed]
- Flach, A.; Dondon, R.C.; Singer, R.B.; Koehler, S.; Amaral, M.D.E.; Marsaioli, A.J. The chemistry of pollination in selected Brazilian maxillariinae orchids: Floral rewards and fragrance. J. Chem. Ecol. 2004, 30, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Petronilho, S.; Rocha, S.M.; Ramírez-Chávez, E.; Molina-Torres, J.; Rios-Chavez, P. Assessment of the terpenic profile of Callistemon citrinus (Curtis) Skeels from Mexico. Ind. Crops Prod. 2013, 46, 369–379. [Google Scholar] [CrossRef]
- Radulović, N.; Dekić, M.; Stojanović-Radić, Z. Chemical composition and antimicrobial activity of the volatile oils of Geranium sanguineum L. and G. robertianum L. (Geraniaceae). Med. Chem. Res. 2012, 21, 601–615. [Google Scholar] [CrossRef]
- Mondello, L.; Costa, R. A new generation of GC capillary columns: SLB-5ms. Report 2006, 20, 17–19. [Google Scholar]
- Silva, I.; Rocha, S.M.; Coimbra, M.A.; Marriott, P.J. Headspace solid-phase microextraction combined with comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry for the determination of volatile compounds from marine salt. J. Chromatogr. A 2010, 1217, 5511–5521. [Google Scholar] [CrossRef]
- Jalali, H.T.; Petronilho, S.; Villaverde, J.J.; Coimbra, M.A.; Domingues, M.R.M.; Ebrahimian, Z.J.; Silvestre, A.J.D.; Rocha, S.M. Assessment of the sesquiterpenic profile of Ferula gummosa oleo-gum-resin (galbanum) from Iran. Contributes to its valuation as a potential source of sesquiterpenic compounds. Ind. Crops Prod. 2013, 44, 185–191. [Google Scholar] [CrossRef]
- Petronilho, S.; Maraschin, M.; Delgadillo, I.; Coimbra, M.A.; Rocha, S.M. Sesquiterpenic composition of the inflorescences of Brazilian chamomile (Matricaria recutita L.): Impact of the agricultural practices. Ind. Crops Prod. 2011, 34, 1482–1490. [Google Scholar] [CrossRef]
- Robinson, C.J. A New Essential Oil—Agonis Fragrans; A Report for the Rural Industries Research and Development Corporation; RIRDC Publication: Barton, ACT, Australia, 2006. [Google Scholar]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Boulton, R. The general relationship between potassium, sodium and pH in grape juice and wine. Am. J. Enol. Vitic. 1980, 32, 182–186. [Google Scholar]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical Changes throughout Grape Berry Development and Fruit and Wine Quality. Food 2007, 1, 1–22. [Google Scholar]
- Pinu, F.; Tumanov, S.; Grose, C.; Raw, V.; Albright, A.; Stuart, L.; Villas-Boas, S.; Martin, D.; Harker, R.; Greven, M. Juice Index: An integrated Sauvignon Blanc grape and wine metabolomics database shows mainly seasonal differences. Metabolomics 2019, 15, 3–21. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Mateus, N.; Machado, J.; de Freitas, V. Development changes of anthocyanins in Vitis vinifera grapes grown in the Douro Valley and concentration in respective wines. J. Sci. Food Agric. 2002, 82, 1689–1695. [Google Scholar] [CrossRef]
- Mateus, N.; Marques, S.; Goncalves, A.; Machado, J.; De Freitas, V. Proanthocyanidin composition of red Vitis vinifera varieties from the Douro valley during ripening: Influence of cultivation altitude. Am. J. Enol. Viticult. 2001, 52, 115–121. [Google Scholar]
- López-Tamames, E.; Carro-Marinõ, N.; Gunata, Y.Z.; Sapis, C.; Baumes, R.; Bayonove, C. Potential Aroma in Several Varieties of Spanish Grapes. J. Agric. Food Chem. 1997, 45, 1729–1735. [Google Scholar] [CrossRef]
- Welke, J.E.; Manfroi, V.; Zanus, M.; Lazarotto, M.; Zini, C.A. Characterization of the volatile profile of Brazilian Merlot wines through comprehensive two dimensional gas chromatography time-of-flight mass spectrometric detection. J. Chromatogr. A 2012, 1226, 124–139. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gandara, J. Wine Aroma Compounds in Grapes: A Critical Review. Crit. Rev. Food Sci. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. The Chemistry of Wine and Stabilization and Treatments. In Handbook of Enology; Ribéreau-Gayon, P., Ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2006; pp. 205–230. [Google Scholar]
- Moreno-Arribas, M.V.; Polo, C. Wine Chemistry and Biochemistry; Springer Science+Business Media, LLC: New York, USA, 2009. [Google Scholar]
- Jackson, R.S. Wine Science: Principles and Applications, 4th ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Marais, I. Terpenes in the aroma of grapes and wines: A review. S. Afr. J. Enol. Vitic. 1983, 4, 49–60. [Google Scholar] [CrossRef]
- Pinho, P.G.; Falqué, E.; Castro, M.; Silva, H.O.; Machado, B.; Ferreira, A.C.S. Further Insights into the Floral Character of Touriga Nacional Wines. J. Food Sci. 2007, 72, S396–S401. [Google Scholar] [CrossRef]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, M.A.; Rocha, S.M. Revealing the Usefulness of Aroma Networks to Explain Wine Aroma Properties: A Case Study of Portuguese Wines. Molecules 2020, 25, 272. [Google Scholar] [CrossRef] [Green Version]
- Bakker, J.; Clarke, J.R. Wine Flavour Chemistry, 2nd ed.; Wiley-Blackwell, Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2012. [Google Scholar]
- Goto-Yamamoto, N.; Mori, K.; Numata, M.; Koyama, K.; Kitayama, M. Effects of Temperature and Water Regimes on Flavonoid Contents and Composition in the Skin of Red-Wine Grapes. J. Int. Sci. Vigne Vin. 2009, 43, 75–80. [Google Scholar]
- Spayd, S.; Tarara, J.; Mee, D.; Ferguson, J. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar]
- Keller, M.; Smith, J.; Bondada, B. Ripening grape berries remain hydraulically connected to the shoot. J. Exp. Bot. 2006, 57, 2577–2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skinkis, P.; Bordelon, B.; Butz, E. Effects of Sunlight Exposure on Berry and Wine Monoterpenes and Sensory Characteristics of Traminette. Am. J. Enol. Vitic. 2010, 61, 147–156. [Google Scholar]
- Zhang, H.; Fan, P.; Liu, C.; Wu, B.; Li, S.; Liang, Z. Sunlight exclusion from Muscat grape alters volatile profiles during berry development. Food Chem. 2014, 164, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Smart, R.; Wang, H.; Dambergs, B.; Sparrow, A.; Qian, M.C. Effect of grape bunch sunlight exposure and UV radiation on phenolics and volatile composition of Vitis vinifera L. cv. Pinot noir wine. Food Chem. 2015, 173, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Koundouras, S.; Marinos, V.; Gkoulioti, A.; Kotseridis, Y.; van Leeuwen, C. Influence of vineyard location and vine water status on fruit maturation of nonirrigated cv. Agiorgitiko (Vitis vinifera L.). Effects on wine phenolic and aroma components. J. Agr. Food Chem. 2006, 54, 5077–5086. [Google Scholar] [CrossRef] [PubMed]
- Bondada, B.; Keller, M. Not All Shrivels Are Created Equal—Morpho-Anatomical and Compositional Characteristics Differ among Different Shrivel Types That Develop during Ripening of Grape (Vitis vinifera L.) Berries. Am. J. Plant Sci. 2012, 3, 879–898. [Google Scholar] [CrossRef] [Green Version]
- Van Leeuwen, C.; Seguin, G. The concept of terroir in viticulture. J. Wine Res. 2006, 17, 1–10. [Google Scholar] [CrossRef]










| Vineyard Parcels Characteristics 1 | ||||||
|---|---|---|---|---|---|---|
| Grape Varieties | Designation | Soil Type | Altitude (m) | Environment | Orientation | Sunlight Exposure |
| White | ||||||
| Arinto (AR) | AR-VA1 | clayey | 50 | open space | North–South | West |
| AR-VA2 | clay–sandy | 70 | near to pine trees | North–South | West | |
| AR-SM1 | clay–calcareous | 50 | open space | East–West | South and West | |
| Bical (BI) | BI-VA1 | clayey | 70 | near to pine trees | North–South | West |
| BI-VA2 | clay–calcareous | 70 | open space | North–South | West | |
| BI-SM1 | clay–sandy | 90 | open space | East–West | South and West | |
| Sauvignon Blanc (SB) | SB-SM1 | clayey | 70 | open space | North–South | South and West |
| SB-SM2 | clay–calcareous | 50 | open space | North–South | South and West | |
| SB-SM3 | clay–sandy | 70 | open space | North–South | South and West | |
| Red | ||||||
| Baga (BA) | BA-VA1 | clayey | 70 | near to pine trees | North–South | South and West |
| BA-VA2 | clay–calcareous | 50 | open space | North–South | South and West | |
| BA-SM1 | clay–sandy | 50 | open space | North–South | South and West | |
| Castelão (CA) | CA-SM1 | clay–calcareous | 70 | open space | North–South | South and West |
| CA-SM2 | clayey | 60 | open space | North–South | South and West | |
| CA-SM3 | clay–sandy | 60 | open space | North–South | South and West | |
| Touriga Nacional (TN) | TN-SM1 | clayey | 50 | open space | North–South | South and West |
| TN-SM2 | clay–calcareous | 70 | open space | North–South | South and West | |
| TN-SM3 | clay–sandy | 50 | open space | North–South | South and West | |
| Vineyard Parcels 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | 2010 | 2011 | 2012 | 2010 | 2011 | 2012 | 2010 | 2011 | 2012 |
| AR-VA1 | AR-VA2 | AR-SM1 | |||||||
| Berry weight (g) | 1.3 (5) 3 | 1.5 (4) | 1.5 (3) | 1.1 (5) | 1.4 (3) | 1.5 (3) | 1.0 (7) | 1.5 (3) | 1.4 (3) |
| pH | 2.7 (2) | 3.2 (1) | 3.2 (0) | 2.9 (1) | 3.2 (0) | 3.1 (1) | 2.7 (1) | 2.9 (1) | 3.1 (0) |
| Acidity (g tartaric acid L−1) | 7.6 (1) | 6.5 (2) | 6.6 (3) | 7.8 (1) | 5.7 (2) | 6.5 (3) | 7.4 (2) | 7.9 (2) | 6.3 (4) |
| Sugar content (g L−1) | 214.8 (1) | 219.3 (1) | 197.8 (3) | 221.0 (1) | 226.1 (2) | 206.3 (3) | 210.0 (1) | 172.3 (1) | 187.0 (4) |
| Phenolic content (mg GAE L−1) | 319.1 (7) | 230.5 (7) | 251.5 (4) | 366.1 (2) | 321.0 (4) | 332.8 (1) | 374.4 (1) | 276.7 (9) | 291.6 (2) |
| Antiradical activity (% DPPHrem) | 85.2 (1) | 84.8 (2) | 88.2 (1) | 68.6 (1) | 70.1 (2) | 80.0 (1) | 70.0 (3) | 73.8 (2) | 82.8 (3) |
| Glycosidically linked content (μg L−1) 2 | 78.1 (11) | 35.6 (12) | 41.9 (10) | 138.0 (10) | 56.7 (14) | 63.7 (10) | 111.3 (10) | 43.9 (13) | 52.9 (12) |
| BI-VA1 | BI-VA2 | BI-SM1 | |||||||
| Berry weight (g) | 1.4 (1.0) | 1.4 (1) | 1.6 (1) | 1.2 (2) | 1.3 (4) | 1.6 (3) | 1.4 (3) | 1.7 (3) | 1.8 (3) |
| pH | 2.9 (2) | 3.2 (1) | 3.2 (1) | 2.9 (0) | 3.1 (0) | 3.2 (1) | 3.2 (0) | 3.0 (1) | 3.1 (1) |
| Acidity (g tartaric acid L−1) | 5.7 (1) | 7.1 (1) | 5.8 (2) | 6.1 (1) | 5.9 (1) | 5.5 (3) | 4.2 (1) | 6.8 (2) | 5.6 (1) |
| Sugar content (g L−1) | 173.4 (1) | 215.3 (1) | 208.5 (1) | 170.0 (1) | 214.8 (1) | 209.7 (1) | 187.0 (1) | 207.4 (1) | 193.2 (1) |
| Phenolic content (mg GAE L−1) | 294.1 (3) | 197.5 (2) | 214.1 (3) | 304.4 (3) | 283.5 (4) | 262.0 (2) | 333.9 (2) | 248.7 (2) | 268.8 (5) |
| Antiradical activity (% DPPHrem) | 81.5 (3) | 89.9 (3) | 89.7 (2) | 84.4 (2) | 81.1 (3) | 86.1 (2) | 71.7 (1) | 81.6 (3) | 83.3 (1) |
| Glycosidically linked content (μg L−1) | 118.3 (11) | 69.3 (10) | 73.1 (13) | 158.3 (10) | 97.6 (12) | 102.1 (13) | 185.5 (10) | 108.6 (10) | 114.1 (13) |
| SB-SM1 | SB-SM2 | SB-SM3 | |||||||
| Berry weight (g) | 1.3 (6) | 1.4 (7) | 1.6 (3) | 1.6 (2) | 1.8 (3) | 1.9 (2) | 1.5 (2) | 1.5 (3) | 1.7 (3) |
| pH | 3.2 (0) | 3.1 (1) | 3.0 (0) | 3.2 (0) | 3.0 (1) | 3.0 (1) | 3.1 (0) | 3.0 (1) | 3.0 (1) |
| Acidity (g tartaric acid L−1) | 6.6 (1) | 7.6 (2) | 7.1 (2) | 5.2 (1) | 7.1 (4) | 6.9 (1) | 6.9 (2) | 7.6 (2) | 7.2 (3) |
| Sugar content (g L−1) | 243.1 (1) | 247.6 (1) | 208.5 (3) | 204.6 (0) | 241.4 (1) | 201.7 (2) | 226.7 (1) | 247.6 (1) | 213.6 (2) |
| Phenolic content (mg GAE L−1) | 255.9 (4) | 236.9 (9) | 276.0 (7) | 403.6 (6) | 283.5 (6) | 315.1 (6) | 467.6 (3) | 357.4 (6) | 365.1 (8) |
| Antiradical activity (% DPPHrem) | 72.6 (2) | 79.9 (2) | 83.1 (2) | 68.9 (1) | 73.5 (3) | 77.6 (1) | 62.7 (2) | 66.4 (2) | 74.2 (2) |
| Glycosidically linked content (μg L−1) | 155.5 (10) | 91.2 (11) | 109.1 (11) | 227.9 (10) | 98.9 (10) | 117.7 (11) | 278.6 (12) | 107.1 (8) | 127.5 (10) |
| BA-VA1 | BA-VA2 | BA-SM1 | |||||||
| Berry weight (g) | 1.3 (4) | 1.9 (1) | 1.7 (4) | 1.7 (3) | 1.9 (4) | 2.0 (3) | 2.1 (4) | 1.9 (4) | 1.8 (4) |
| pH | 3.1 (0) | 3.2 (0) | 3.1 (0) | 3.1 (1) | 3.1 (2) | 2.9 (1) | 3.2 (2) | 3.2 (1) | 3.1 (1) |
| Acidity (g tartaric acid L−1) | 5.2 (2) | 6.3 (2) | 5.8 (3) | 4.1 (3) | 6.4 (2) | 5.3 (6) | 5.3 (1) | 7.0 (3) | 5.6 (5) |
| Sugar content (g L−1) | 189.8 (2) | 196.6 (1) | 176.8 (3) | 214.8 (2) | 202.3 (1) | 163.8 (2) | 176.8 (1) | 190.4 (2) | 175.7 (3) |
| Phenolic content (mg GAE L−1) | 811.0 (3) | 631.4 (17) | 594.8 (15) | 1270.1 (15) | 869.3 (11) | 726.1 (8) | 686.0 (12) | 517.0 (20) | 494.1 (11) |
| Antiradical activity (% DPPHrem) | 80.9 (2) | 85.6 (2) | 82.0 (4) | 53.5 (2) | 70.3 (3) | 73.8 (1) | 81.0 (3) | 88.6 (2) | 89.6 (2) |
| Glycosidically linked content (μg L−1) | 51.1 (12) | 35.0 (12) | 33.6 (12) | 61.4 (11) | 40.9 (12) | 40.5 (11) | 25.0 (11) | 22.3 (14) | 22.9 (11) |
| CA-SM1 | CA-SM2 | CA-SM3 | |||||||
| Berry weight (g) | 1.8 (3) | 2.0 (1) | 2.0 (2) | 1.9 (4) | 1.8 (6) | 1.8 (4) | 1.9 (3) | 1.9 (4) | 1.9 (3) |
| pH | 3.3 (1) | 3.2 (1) | 3.1 (1) | 3.2 (0) | 3.1 (1) | 3.1 (0) | 3.3 (1) | 3.2 (0) | 3.2 (2) |
| Acidity (g tartaric acid L−1) | 6.0 (2) | 7.2 (2) | 7.5 (3) | 5.2 (2) | 5.8 (2) | 6.3 (4) | 5.5 (1) | 5.7 (3) | 7.1 (1) |
| Sugar content (g L−1) | 221.0 (2) | 196.1 (1) | 181.9 (4) | 241.4 (1) | 218.7 (1) | 186.4 (4) | 218.2 (2) | 193.2 (1) | 170.0 (4) |
| Phenolic content (mg GAE L−1) | 1053.4 (4) | 704.9 (13) | 694.1 (14) | 864.0 (4) | 621.6 (20) | 627.5 (16) | 826.1 (5) | 635.2 (16) | 522.0 (12) |
| Antiradical activity (% DPPHrem) | 64.3 (3) | 69.9 (1) | 70.2 (2) | 68.7 (2) | 72.0 (1) | 75.9 (3) | 77.4 (2) | 81.9 (3) | 89.4 (4) |
| Glycosidically linked content (μg L−1) | 56.3 (10) | 37.6 (11) | 43.0 (10) | 48.8 (13) | 31.7 (11) | 35.1 (13) | 40.9 (7) | 24.2 (10) | 24.4 (16) |
| TN-SM1 | TN-SM2 | TN-SM3 | |||||||
| Berry weight (g) | 1.9 (2) | 1.7 (3) | 2.0 (3) | 1.6 (6) | 1.6 (3) | 1.7 (5) | 1.9 (1) | n.a. 4 | 2.0 (5) |
| pH | 3.2 (0) | 3.2 (0) | 3.2 (1) | 3.3 (2) | 3.3 (1) | 3.3 (1) | 3.3 (1) | n.a. | 3.3 (0) |
| Acidity (g tartaric acid L−1) | 4.4 (3) | 5.7 (2) | 5.8 (2) | 4.2 (3) | 5.5 (3) | 5.3 (6) | 4.3 (2) | n.a. | 6.1 (7) |
| Sugar content (g L−1) | 200.1 (2) | 193.8 (1) | 187.6 (3) | 254.2 (6) | 205.1 (2) | 201.2 (4) | 199.4 (3) | n.a. | 184.7 (2) |
| Phenolic content (mg GAE L−1) | 747.3 (2) | 948.9 (13) | 646.5 (13) | 1341.3 (2) | 1339.8 (9) | 1121.4 (9) | 852.0 (11) | n.a. | 501.6 (14) |
| Antiradical activity (% DPPHrem) | 78.3 (3) | 85.0 (1) | 81.2 (1) | 51.9 (1) | 58.4 (2) | 66.9 (1) | 70.0 (3) | n.a. | 86.3 (1) |
| Glycosidically linked content (μg L−1) | 50.8 (13) | 38.2 (12) | 40.4 (10) | 67.8 (12) | 43.3 (15) | 46.6 (13) | 34.6 (13) | n.a. | 29.1 (11) |
| Grape Varieties | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1Dtr(s), 2Dtr(s) 1 | Compound | CAS Number | Formula | RIcalc. 2 | RIlit. 3 | Ref. RIlit. 4 | AR | BI | SB | BA | CA | TN |
| C6 compounds | ||||||||||||
| 194, 0.640 | Hexanal | 66-25-1 | C6H12O | 801 | 801 | [28] | F ** | F | F | F | F | F |
| 206, 0.630 | 3-Hexenal | 6789-80-6 | C6H10O | 805 | 807 | [28] | F | F | F | F | F | F |
| 230, 0.651 | 2-Hexenal | 6728-26-3 | C6H10O | 851 | 855 | [29] | F | F | F | F | F | F |
| 242, 0.630 | 3-Hexen-1-ol | 928-96-1 | C6H12O | 858 | 861 | [28] | F | F | F | F | F | F |
| 248, 1.076 | 2-Hexen-1-ol | 928-95-0 | C6H12O | 864 | 861 | [29] | F | F | F | F | F | F |
| 266, 0.903 | 1-Hexanol | 111-27-3 | C6H14O | 876 | 877 | [29] | F | F | F | F | F | F |
| 296, 0.930 | 2,4-Hexadienal | 142-83-6 | C6H8O | 914 | 914 | [30] | F | F | F | F | F | F |
| Aromatic alcohols | ||||||||||||
| 420, 3.014 | Benzyl Alcohol | 100-51-6 | C7H8O | 1048 | 1044 | [31] | F | F | F | F | F | F |
| 446, 1.426 | α,α-Dimethyl Benzyl alcohol | 617-94-7 | C9H12O | 1089 | 1091 | [28] | F | F | F | F | F | F |
| 470, 1.960 | 2-Phenylethanol | 60-12-8 | C8H10O | 1115 | 1120 | [28] | F | F | F | F | F | F |
| C9 Norisoprenoid | ||||||||||||
| 506, 0.761 | Norinone | 38651-65-9 | C9H14O | 1142 | 1183 | [32] | F | F | F | |||
| Monoterpenic compounds | ||||||||||||
| 314, 0.440 | α-Pinene | 80-56-8 | C10H16 | 938 | 941 | [33] | F, G | F, G | F, G | F, G | F, G | F |
| 338, 0.480 | m/z 91, 119, 77, 185 (hydrocarbon) | - | - | 972 | - | - | F | F | F | |||
| 344, 0.457 | β-Pinene * | 18172-67-3 | C10H16 | 988 | 987 | [33] | F, G | F, G | F | F, G | F, G | F |
| 356, 0.570 | β-Myrcene | 123-35-3 | C10H16 | 1001 | 1008 | [33] | F | F | F | F, G | G | |
| 362, 0.520 | 3-Carene | 13466-78-9 | C10H16 | 1007 | 1020 | [33] | G | F | F, G | F | ||
| 368, 0.790 | α-Phellandrene | 99-83-2 | C10H16 | 1013 | 1010 | [34] | F | |||||
| 392, 0.405 | m-Cymene | 535-77-3 | C10H14 | 1025 | 1027 | [33] | F | F, G | F | F | F | |
| 398, 0.476 | Limonene * | 138-86-3 | C10H16 | 1028 | 1035 | [33] | F, G | F, G | F, G | F | F | F, G |
| 404, 0.476 | 1,8-Cineole | 470-82-6 | C10H18O | 1034 | 1039 | [33] | F, G | F, G | F, G | F | F | F |
| 416, 0.560 | β-Ocimene | 3779-61-1 | C10H16 | 1045 | 1043 | [33] | F | F | F | F | ||
| 428, 0.678 | Linalool oxide (isomer 1) | - | C10H18O2 | 1071 | 1078 | [33] | F, G | F, G | F, G | F | F | F |
| 434, 0.727 | Dihydromyrcenol | 53219-21-9 | C10H20O | 1073 | 1076 | [32] | F | F | F | F | F | F |
| 440, 0.560 | α-Terpinolene | 586-62-9 | C10H16 | 1076 | 1097 | [33] | F | G | F | F | F | F |
| 440, 0.790 | Linalool oxide (isomer 2) | - | C10H18O2 | 1076 | 1097 | [33] | G | F | F | F | ||
| 446, 0.700 | Dihydrolinalool | 78-69-3 | C10H22O | 1088 | 1101 | [28] | F | F | F | F | F | F |
| 452, 0.746 | Linalool * | 78-70-6 | C10H18O | 1096 | 1108 | [33] | F, G | F, G | F, G | F | F | F, G |
| 464, 0.600 | Rose oxide (isomer 1) | - | C10H18O | 1107 | 1117 | [33] | F | F | F | F | F | |
| 464, 0.844 | Fenchol | 22627-95-8 | C10H18O | 1108 | 1118 | [33] | F | F | F | F | F | |
| 470, 0.646 | Hotrienol | 53834-70-1 | C10H16O | 1113 | 1122 | [33] | F | F, G | F | F | F | F |
| 470, 0.780 | Camphenal | 4501-58-0 | C10H14O | 1114 | 1130 | [33] | F | F | F | |||
| 476, 0.770 | Rose oxide (isomer 2) | - | C10H18O | 1118 | 1130 | [33] | F | |||||
| 482, 0.890 | 1-Terpineol | 586-82-3 | C10H18O | 1120 | 1127 | [35] | F | F | ||||
| 488, 0.690 | Cosmene | 460-01-5 | C10H14 | 1122 | 1134 | [36] | F | F | ||||
| 494, 1.050 | Pinocarveol | 547-61-5 | C10H16O | 1130 | 1142 | [33] | F | F | ||||
| 500, 0.970 | β-Terpineol | 138-87-4 | C10H18O | 1136 | 1150 | [33] | F | F | ||||
| 506, 1.190 | Pinocarvone | 34-41-3 | C10H14O | 1140 | 1164 | [33] | F | |||||
| 512, 0.635 | Nerol oxide | 1786-08-9 | C10H16O | 1151 | 1172 | [33] | F | F | F | F | F | F |
| 518, 0.834 | Ocimenol | 5986-38-9 | C10H18O | 1166 | 1179 | [33] | F | F | F | F | ||
| 518, 1.200 | m/z 68, 94, 79, 109 (alcohol) | - | - | 1167 | - | - | F | F | F | |||
| 524, 0.860 | Borneol | 507-70-0 | C10H18O | 1169 | 1172 | [33] | F | F | F, G | F | F | F |
| 530, 0.884 | p-Mentha-1,5-dien-8-ol | 1686-20-0 | C10H16O | 1171 | 1172 | [33] | F | F | ||||
| 530, 0.984 | Menthol * | 1490-04-6 | C10H20O | 1175 | 1170 | [37] | F | F | F | F | F | F |
| 536, 0.715 | Terpinen-4-ol | 562-74-3 | C10H18O | 1183 | 1181 | [33] | F | F, G | F, G | F | F | F |
| 536, 1.269 | p-Cymen-8-ol | 1197-01-9 | C10H14O | 1184 | 1203 | [33] | F | F | F | F | F | F |
| 542, 0.835 | α-Terpineol * | 98-55-5 | C10H18O | 1195 | 1206 | [33] | F | F, G | F, G | F | F | F |
| 548, 0.850 | Dihydrocarvone | 7764-50-3 | C10H16O | 1197 | 1211 | [33] | G | G | F | |||
| 554, 0.900 | Safranal | 116-26-7 | C10H14O | 1199 | 1201 | [30] | F | |||||
| 560, 0.850 | Verbenone | 80-57-9 | C10H14O | 1214 | 1214 | [33] | F | F, G | F, G | F | F | F |
| 566, 0.703 | p-Menth-1-en-9-al | 29548-14-9 | C10H16O | 1217 | 1219 | [33] | F | F, G | F | F | F | F |
| 572, 1.340 | 2-Hydroxycineole | 92999-78-5 | C10H18O2 | 1219 | 1237 | [38] | F | F | ||||
| 578, 0.700 | m/z 93, 121, 119, 136 (alcohol) | - | - | 1224 | - | - | G | F | ||||
| 584, 0.873 | Geraniol (isomer 1) * | - | C10H18O | 1235 | 1235 | [33] | F | F | F, G | F | F | F |
| 584, 0.943 | β-Citronellol * | 106-22-9 | C10H20O | 1237 | 1237 | [33] | F | F | F, G | F | F | F |
| 590, 0.737 | Geraniol (isomer 2) * | - | C10H18O | 1244 | 1242 | [33] | F | F, G | F, G | F | F | F |
| 596, 0.976 | Citral (isomer 1) | - | C10H16O | 1247 | 1245 | [33] | F | F, G | F, G | F | F | F |
| 602, 0.815 | Carvone * | 99-49-0 | C10H14O | 1251 | 1245 | [33] | F | F | F, G | F | F | F |
| 626, 0.775 | Citral (isomer 2) | - | C10H16O | 1274 | 1287 | [32] | F, G | F, G | F, G | F | F | F |
| C13 Norisoprenoids | ||||||||||||
| 566, 0.532 | m/z 159, 91, 131 (hydrocarbon) | - | - | 1216 | - | - | F, G | F | F | F | F | |
| 602, 0.660 | α-Ionene | 475-03-6 | C13H19 | 1250 | 1261 | [31] | F | |||||
| 620, 0.595 | Vitispirane | 65416-59-3 | C13H20O | 1286 | 1287 | [31] | F | F | F | F | F | F |
| 632, 0.517 | Theaspirane (isomer 1) | - | C13H22O | 1302 | 1305 | [31] | F | F | F | F | F | |
| 644, 0.528 | Theaspirane (isomer 2) | - | C13H22O | 1323 | 1322 | [31] | F | F | F | F | F | F |
| 668, 0.790 | TDN(1,2-dihydro-1,1,6-trimethyl-naphthalene) | 30364-38-6 | C13H16 | 1357 | 1361 | [31] | F | F | ||||
| 674, 0.681 | β-Damascenone (isomer 1) | - | C13H18O | 1369 | 1364 | [31] | F | F | F | F | F | F |
| 680, 0.840 | m/z 142, 157, 115 (ketone) | - | - | 1371 | - | - | F | F | F | |||
| 700, 0.702 | β-Damascenone (isomer 2) | - | C13H18O | 1383 | 1385 | [31] | F | F | F | F | F | F |
| 724, 0.750 | Hydroxydihydroedulan | - | C13H22O2 | 1446 | 1446 | [39] | F | F | ||||
| 736, 0.648 | Geranylacetone * | 3796-70-1 | C13H22O | 1455 | 1454 | [29] | F, G | F, G | F | F, G | F, G | F, G |
| 742, 0.850 | 5,6-Epoxy-β-ionone | 23267-57-4 | C13H20O2 | 1463 | 1460 | [30] | F | F | F | F | F | F |
| 760, 0.868 | 3,4-Dehydro-β-ionone | 1203-08-3 | C13H18O | 1474 | 1483 | [31] | F | F | F | F | F | |
| 778, 0.635 | α-Iso-methyl ionone | 127-51-5 | C14H22O | 1485 | 1471 | [40] | F | F | F | F | F | F |
| 784, 0.717 | β-Ionone * | 79-77-6 | C13H20O | 1488 | 1494 | [41] | F | F, G | F | F | F | F |
| 900, 0.894 | Methyl dihydrojasmonate | 24851-98-7 | C13H22O3 | 1661 | 1650 | [40] | F, G | F, G | F, G | F, G | F, G | F, G |
| Sesquiterpenic compounds | ||||||||||||
| 650, 0.521 | δ-Elemene | 20307-84-0 | C15H24 | 1329 | 1330 | [42] | F | F | F | |||
| 656, 0.583 | α-Longipinene | 5989-08-2 | C15H24 | 1337 | 1359 | [42] | F | F | F | |||
| 680, 0.469 | α-Copaene | 3856-25-5 | C15H24 | 1371 | 1375 | [42] | F | F | F | F | ||
| 686, 0.510 | β-Bourbonene | 5208-59-3 | C15H24 | 1379 | 1379 | [42] | F | F | ||||
| 712, 0.508 | Longifolene | 475-20-7 | C15H24 | 1414 | 1395 | [42] | F | F | F | |||
| 718, 0.481 | β-Caryophyllene | 87-44-5 | C15H24 | 1418 | 1417 | [42] | F | |||||
| 724, 0.541 | α-Humulene | 6753-98-6 | C15H24 | 1445 | 1450 | [42] | F | F | ||||
| 756, 0.630 | Aromadendrene | 489-39-4 | C15H24 | 1477 | 1478 | [38] | F | F | F | F | F | F |
| 762, 0.450 | α-Muurolene | 31983-22-9 | C15H24 | 1485 | 1490 | [42] | F | F | ||||
| 790, 0.660 | α-Farnesene | 502-61-4 | C15H24 | 1501 | 1505 | [43] | F, G | F, G | F | F, G | F, G | |
| 796, 0.525 | γ-Cadinene | 39029-41-9 | C15H24 | 1504 | 1511 | [42] | F | F, G | ||||
| 808, 0.630 | Calamenene | 483-77-2 | C15H22 | 1514 | 1520 | [42] | F | F | F | F | F | |
| 826, 0.629 | α-Calacorene | 21391-99-1 | C15H20 | 1555 | 1554 | [42] | F | F | F, G | F | F | |
| 832, 0.880 | Nerolidol | 7212-44-4 | C15H26O | 1560 | 1568 | [42] | F | F | F | F | F | F |
| 844, 0.810 | Epiglobulol | 88728-58-9 | C15H26O | 1579 | 1582 | [44] | F | |||||
| 850, 0.751 | Globulol | 489-41-8 | C15H26O | 1594 | 1592 | [38] | F | G | F | |||
| 862, 0.726 | Caryophyllene oxide | 1139-30-6 | C15H24O | 1605 | 1601 | [42] | F | F | F | |||
| 886, 0.690 | β-Eudesmol | 77-53-2 | C15H26O | 1642 | 1642 | [42] | F | F | F | F | F | F |
| 912, 0.654 | m/z 119, 91, 191, 109 (alcohol) | - | - | 1675 | - | - | F | F, G | F, G | G | F, G | F, G |
| 942, 0.820 | Farnesal | 502-67-0 | C15H24O | 1731 | 1724 | [42] | G | F, G | G | |||
| 1036, 0.671 | Ledene oxide | - | C15H24O | 1873 | 1867 | [42] | G | G | G | G | G | |
| Diterpenoid | ||||||||||||
| 1116, 0.929 | Phytol | 596-84-9 | C20H34O | 2021 | 2022 | [38] | F | F | F | |||
| Variety | Factors | p-Value (2000 Permutations) | Explained Variance (%) |
|---|---|---|---|
| Arinto | Harvest year | <0.0005 | 62.2 |
| Vineyard parcel | 0.0390 | 14.9 | |
| Bical | Harvest year | <0.0005 | 53.8 |
| Vineyard parcel | 0.0135 | 17.1 | |
| Sauvignon Blanc | Harvest year | <0.0005 | 68.2 |
| Vineyard parcel | >0.05 * | 11.9 | |
| Baga | Harvest year | <0.0005 | 61.4 |
| Vineyard parcel | 0.040 | 14.8 | |
| Castelão | Harvest year | <0.0005 | 66.7 |
| Vineyard parcel | 0.041 | 15.5 | |
| Touriga Nacional | Harvest year | <0.0005 | 59.3 |
| Vineyard parcel | 0.0105 | 18.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petronilho, S.; Rudnitskaya, A.; Coimbra, M.A.; Rocha, S.M. Comprehensive Study of Variety Oenological Potential Using Statistic Tools for the Efficient Use of Non-Renewable Resources. Appl. Sci. 2021, 11, 4003. https://doi.org/10.3390/app11094003
Petronilho S, Rudnitskaya A, Coimbra MA, Rocha SM. Comprehensive Study of Variety Oenological Potential Using Statistic Tools for the Efficient Use of Non-Renewable Resources. Applied Sciences. 2021; 11(9):4003. https://doi.org/10.3390/app11094003
Chicago/Turabian StylePetronilho, Sílvia, Alisa Rudnitskaya, Manuel A. Coimbra, and Sílvia M. Rocha. 2021. "Comprehensive Study of Variety Oenological Potential Using Statistic Tools for the Efficient Use of Non-Renewable Resources" Applied Sciences 11, no. 9: 4003. https://doi.org/10.3390/app11094003
APA StylePetronilho, S., Rudnitskaya, A., Coimbra, M. A., & Rocha, S. M. (2021). Comprehensive Study of Variety Oenological Potential Using Statistic Tools for the Efficient Use of Non-Renewable Resources. Applied Sciences, 11(9), 4003. https://doi.org/10.3390/app11094003