Total Phenolic, Anthocyanins HPLC-DAD-MS Determination and Antioxidant Capacity in Black Grape Skins and Blackberries: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
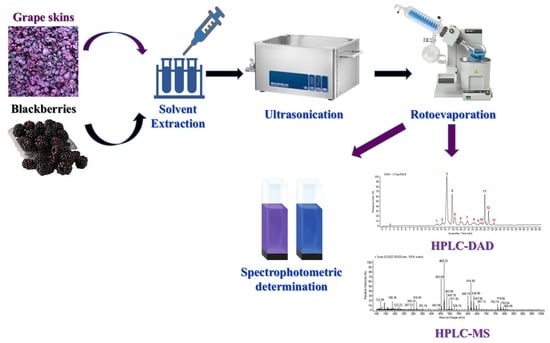
2.1. Raw Materials and Extraction Procedures
2.2. Total Polyphenolic Content
2.3. HPLC-DAD-MS Method
2.4. Monomeric Anthocyanin and Polymeric Colour Measurement
2.5. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.6. Statistical Analysis
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insights (Review). Int. J. Funct. Nutr. 2020, 1, 1. [Google Scholar] [CrossRef]
- Mileo, A.M.; Nisticò, P.; Miccadei, S. Polyphenols: Immunomodulatory and Therapeutic Implication in Colorectal Cancer. Front. Immunol. 2019, 10, 729. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols From Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. Polyphen. Plants 2019, 243–259. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.-V.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- An, C.Y.; Hossain, M.; Alam, F.; Islam, A.; Khalil, I.; Alam, N.; Gan, S.H. Efficiency of Polyphenol Extraction from Artificial Honey Using C 18 Cartridges and Amberlite® XAD-2 Resin: A Comparative Study. J. Chem. 2016, 2016, 8356739. [Google Scholar] [CrossRef] [Green Version]
- Kammerer, D. Anthocyanins. In Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color; Woodhead Publishing: Sawston, UK, 2016; pp. 61–80. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pascual-Teresa, S.; Sánchez-Ballesta, M.T.; Garcia-Viguera, C.; Pascual-Teresa, S. Anthocyanins. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1803–1819. [Google Scholar] [CrossRef]
- Paun, N.; Niculescu, V.; Tamaian, R.; Miricioiu, M. Functionalized Mesoporous Silica Nanoparticles as Novel Systems for Natural Anthocyanins Stability Enhancement. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, Albena, Bulgary, 29 June–5 July 2017; Volume 17. [Google Scholar]
- Jordheim, M.; Calcott, K.; Gould, K.S.; Davies, K.M.; Schwinn, K.E.; Andersen, O.M. High concentrations of aromatic acylated anthocyanins found in cauline hairs in Plectranthus ciliates. Phytochemistry 2016, 128, 27–34. [Google Scholar] [CrossRef]
- Flamini, R.; Mattivi, F.; De Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced Knowledge of Three Important Classes of Grape Phenolics: Anthocyanins, Stilbenes and Flavonols. Int. J. Mol. Sci. 2013, 14, 9651. [Google Scholar] [CrossRef]
- Niculescu, V.-C.; Paun, N.; Ionete, R.-E. The Evolution of Polyphenols from Grapes to Wines. In Grapes and Wines-Advances in Production, Processing, Analysis and Valorization; Jordao, A.M., Cosme, F., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Krga, I.; Tamaian, R.; Mercier, S.; Boby, C.; Monfoulet, L.-E.; Glibetic, M.; Morand, C.; Milenkovic, D. Anthocyanins and their gut metabolites attenuate monocyte adhesion and transendothelial migration through nutrigenomic mechanisms regulating endothelial cell permeability. Free Radic. Biol. Med. 2018, 124, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Horbowicz, M.; Kosson, R.; Grzesiuk, A.; Dębski, H. Anthocyanins of Fruits and Vegetables-Their Occurrence, Analysis and Role in Human Nutrition. J. Fruit Ornam. Plant Res. 2008, 68, 5–22. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Gündoğdu, M.; Kan, T.; Canan, I. Bioactive and antioxidant characteristics of blackberry cultivars from East Anatolia. Turk. J. Agric. For. 2016, 40, 344–351. [Google Scholar] [CrossRef]
- Milivojević, J.; Maksimović, V.; Nikolić, M.; Bogdanović, J.; Maletić, R.; Milatović, D. Chemical and antioxidant properties of cultivated and wild fragaria and rubus berries. J. Food Qual. 2011, 34, 1–9. [Google Scholar] [CrossRef]
- Geana, E.-I.; Popescu, R.; Costinel, D.; Dinca, O.R.; Ionete, R.E.; Stefanescu, I.; Artem, V.; Bala, C. Classification of red wines using suitable markers coupled with multivariate statistic analysis. Food Chem. 2016, 192, 1015–1024. [Google Scholar] [CrossRef]
- Ionete, R.E.; Stegarus, D.I.; Geana, E.I.; Botoran, O.R.; Sandru, C.; Miricioiu, M.G. Characterization and Classification of Romanian Wines by Origin A chemometric approach based on some metals and phenolic composition. Rev. Chim. 2019, 70, 3761–3768. [Google Scholar] [CrossRef]
- Francini, A.; Pintado, M.M.; Manganaris, G.A.; Ferrante, A. Editorial: Bioactive Compounds Biosynthesis and Metabolism in Fruit and Vegetables. Front. Plant Sci. 2020, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Artem, V.; Antoce, A.O.; Geana, E.-I.; Ionete, R.E. Study of the impact of vine cultivation technology on the Feteasca Neagra wine phenolic composition and antioxidant properties. J. Food Sci. Technol. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; De Biaggi, M.; Riondato, I.; Rakotoniaina, E.N.; Beccaro, G.L. New Findings in Prunus padus L. Fruits as a Source of Natural Compounds: Characterization of Metabolite Profiles and Preliminary Evaluation of Antioxidant Activity. Molecules 2018, 23, 725. [Google Scholar] [CrossRef] [Green Version]
- Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, Ł.; Stuper-Szablewska, K. Sambucus Nigra Extracts–Natural Antioxidants and Antimicrobial Compounds. Molecules 2021, 26, 2910. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, G.-I.; Almajano, M.P. Red Fruits: Extraction of Antioxidants, Phenolic Content, and Radical Scavenging Determination: A Review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niessen, W.; Tinke, A. Liquid chromatography-mass spectrometry General principles and instrumentation. J. Chromatogr. A 1995, 703, 37–57. [Google Scholar] [CrossRef]
- Abian, J. The Coupling of Gas and Liquid Chromatography with Mass Spectrometry. J. Mass Spectrom. 1999, 34, 157–168. [Google Scholar] [CrossRef]
- Do, Q.-D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamuela-Raventós, R.M. Folin-Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. Meas. Antioxid. Act. Capacit. 2017, 107–117. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-Visible spectroscopy. Curr. Prot. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Metivier, R.P.; Francis, F.J.; Clydesdale, F.M. Solvent extraction of anthocyanins from wine pomace. J. Food Sci. 1980, 45, 1099–1100. [Google Scholar] [CrossRef]
- Yuan, J.; Li, H.; Tao, W.; Han, Q.; Dong, H.; Zhang, J.; Jing, Y.; Wang, Y.; Xiong, Q.; Xu, T. An effective method for extracting anthocyanins from blueberry based on freeze-ultrasonic thawing technology. Ultrason. Sonochem. 2020, 68, 105192. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Sun, Y.; Zhao, G.; Liao, X.; Hu, X.; Wu, J.; Wang, Z. Optimization of ultrasound-assisted extraction of anthocyanins in red raspberries and identification of anthocyanins in extract using high-performance liquid chromatography–mass spectrometry. Ultrason. Sonochem. 2007, 14, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, R.; Haenen, G.R.; van den Berg, H.; van der Vijgh, W.; Bast, A. The predictive value of the antioxidant capacity of structurally related flavonoids using the Trolox equivalent antioxidant capacity (TEAC) assay. Food Chem. 2000, 70, 391–395. [Google Scholar] [CrossRef]
- Kim, J.-S. Antioxidant Activities of Selected Berries and Their Free, Esterified, and Insoluble-Bound Phenolic Acid Contents. Prev. Nutr. Food Sci. 2018, 23, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Juan, M.-Y.; Chou, C.-C. Enhancement of antioxidant activity, total phenolic and flavonoid content of black soybeans by solid state fermentation with Bacillus subtilis BCRC 14715. Food Microbiol. 2010, 27, 586–591. [Google Scholar] [CrossRef]
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the antioxidant capacity of chickpeas by solid state fermentation with Cordyceps militaris SN-18. J. Funct. Foods 2014, 10, 210–222. [Google Scholar] [CrossRef]
- Ştefănuţ, M.N.; Căta, A.; Pop, R.; Mosoarca, C.; Zamfir, A.D. Anthocyanins HPLC-DAD and MS Characterization, Total Phenolics, and Antioxidant Activity of Some Berries Extracts. Anal. Lett. 2011, 44, 2843–2855. [Google Scholar] [CrossRef]
- Avram, S.; Danciu, C.; Pavel, I.Z.; Ceausu, R.A.; Avram, S.; Dehelean, C.; Raica, M. Polyphenols, Antioxidant Activity and Anti-angiogenic Potential of Red and White Grapes. Rev. Chim. 2016, 67, 382–385. [Google Scholar]
- Gil Lee, S.; Vance, T.M.; Nam, T.G.; Kim, D.-O.; Koo, S.I.; Chun, O.K. Contribution of Anthocyanin Composition to Total Antioxidant Capacity of Berries. Plant Foods Hum. Nutr. 2015, 70, 427–432. [Google Scholar] [CrossRef]









| Raw Material | TC (mg/g) | TA (mg/g) | PC (%) | TAC (µmol TE/g Extract) |
|---|---|---|---|---|
| Blackberry puree (50% EOH) | 25.83 | 18.80 | 15.88 | 264.27 |
| Grape skin puree (50% EOH) | 14.00 | 11.06 | 8.97 | 216.19 |
| Blackberry puree (80% MeOH) | 26.40 | 13.90 | 10.30 | 189.30 |
| Grape skin puree (80% MeOH) | 21.30 | 7.65 | 5.22 | 168.26 |
| Peak Number | RT (min) | m/z | Mass Assignment (M+ Sau [M + H]+) | Abv. * | Anthocyanin Content (%) |
|---|---|---|---|---|---|
| Othello grape skin extract | |||||
| 1 | 13.9 | 633 | Malvidin-Delphinidin (MvDp_ fragment) | M1 | 1.87 |
| 2 | 15.1 | 617 | Malvidin-Cyanidin (MvCy) fragment | M2 | 1.87 |
| 3 | 16.2 | 469/303 | Delphinidin-3-O-glucoside | D1 | 40.64 |
| 4 | 17.6 | 453/287 | Cyanidin-3-O-glucoside | C1 | 17.82 |
| 5 | 18.2 | 453/287 | Cyanidin-3-O-galactoside | C2 | 5.75 |
| 6 | 19.7 | 467/303 | Delphinidin-3-O-glucoside | D2 | 5.44 |
| 7 | 21.5 | 511/303 | Delphinidin-3-(6-O-acetylglucoside) | D3 | 4.25 |
| 8 | 23.2 | 779/287 495 | Malvidin-Cyanidin (MvCy) fragment + glucose (G) Malvidin-3-O-glucoside | M3 | 4.75 |
| 9 | 24.7 | 763 | Cyanidin-3-glucoside-Ethyl-coumaroyl (Cy-3-glc-Ethyl-C) | C3 | 1.16 |
| 10 | 25.1 | 763 617 | Cyanidin-3-glucoside-Ethyl-coumaroyl (Cy-3-glc-Ethyl-C) Malvidin-Cyanidin (MvCy) fragment | C4 | 0.53 |
| 11 | 25.8 | 807 617 | Malvidin-3-glucoside-8- vinyl(epi)catechin (Mv-3-glu-8-vinyl(epi)catechin) Malvidin-Cyanidin (MvCy) fragment | M4 | 9.71 |
| 12 | 26.8 | 601 | Malvidin-3-(6-O-acetylglucoside) pyruvate | M5 | 6.01 |
| 13 | 28.1 | 645 | Malvidin-Petunidin fragment (MvPt) | M6 | 0.16 |
| Blackberry extract | |||||
| 1 | 18.0 | 453/287 | Cyanidin-3-O-glucoside | Cb1 | 86.49 |
| 2 | 19.7 | 437/303 | Delphinidin-3-xyloside | D b1 | 0.26 |
| 3 | 20.7 | 422/287 | Cyanidin-3-O-arabinoside | Cb2 | 6.40 |
| 4 | 21.4 | 540/287 | Cyanidin-3-malonyl-glucoside | Cb3 | 2.15 |
| 5 | 22.9 | 598/287 | Cyanidin-3-rutinoside | Cb4 | 4.57 |
| Blackberries (50% EOH) | TP | TA | PC | TAC |
|---|---|---|---|---|
| TP | 1.000 | 0.728 | 0.615 | 0.977 |
| TA | 1.000 | 0.845 | 0.711 | |
| PC | 1.000 | 0.601 | ||
| TAC | 1.000 | |||
| Grape skins (50% EOH) | TP | TA | PC | TAC |
| TP | 1.000 | 0.790 | 0.641 | 0.648 |
| TA | 1.000 | 0.811 | 0.512 | |
| PC | 1.000 | 0.415 | ||
| TAC | 1.000 | |||
| Blackberries (80% MeOH) | TP | TA | PC | TAC |
| TP | 1.000 | 0.527 | 0.390 | 0.717 |
| TA | 1.000 | 0.741 | 0.734 | |
| PC | 1.000 | 0.544 | ||
| TAC | 1.000 | |||
| Grape skins (80% MeOH) | TP | TA | PC | TAC |
| TP | 1.000 | 0.359 | 0.245 | 0.790 |
| TA | 1.000 | 0.682 | 0.455 | |
| PC | 1.000 | 0.310 | ||
| TAC | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paun, N.; Botoran, O.R.; Niculescu, V.-C. Total Phenolic, Anthocyanins HPLC-DAD-MS Determination and Antioxidant Capacity in Black Grape Skins and Blackberries: A Comparative Study. Appl. Sci. 2022, 12, 936. https://doi.org/10.3390/app12020936
Paun N, Botoran OR, Niculescu V-C. Total Phenolic, Anthocyanins HPLC-DAD-MS Determination and Antioxidant Capacity in Black Grape Skins and Blackberries: A Comparative Study. Applied Sciences. 2022; 12(2):936. https://doi.org/10.3390/app12020936
Chicago/Turabian StylePaun, Nadia, Oana Romina Botoran, and Violeta-Carolina Niculescu. 2022. "Total Phenolic, Anthocyanins HPLC-DAD-MS Determination and Antioxidant Capacity in Black Grape Skins and Blackberries: A Comparative Study" Applied Sciences 12, no. 2: 936. https://doi.org/10.3390/app12020936
APA StylePaun, N., Botoran, O. R., & Niculescu, V. -C. (2022). Total Phenolic, Anthocyanins HPLC-DAD-MS Determination and Antioxidant Capacity in Black Grape Skins and Blackberries: A Comparative Study. Applied Sciences, 12(2), 936. https://doi.org/10.3390/app12020936