Directionally Solidified Cobalt-Doped MgO-MgAl2O4 Eutectic Composites for Selective Emitters
Abstract
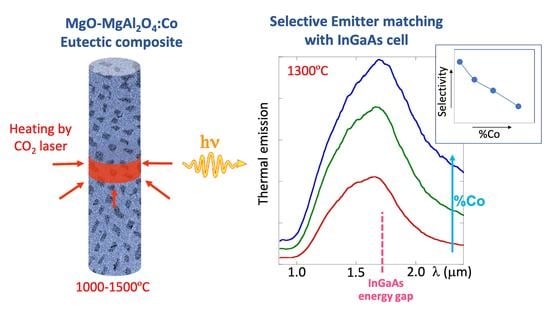
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Microstructure
3.2. Thermal Emission
3.3. Room Temperature Absorption and Emittance
4. Discussion
5. Conclusions
- -
- Most of the cobalt was introduced in the material sites in the MgO phase. Around 30% of Cobalt located in the MgAl2O4 phase is responsible for the optical properties of the material which are relevant to the thermal emission, due to the much larger optical activity of cobalt in tetrahedrally coordinated sites.
- -
- The materials have a high emittance band around 1500–1700 nm, even for low cobalt ion concentration, which would make the material appropriate as selective emitter for thermophotovoltaic systems based on InGaAs detector. Very low cobalt concentration (0.04% at Co; 0.2 wt % CoO) is enough to produce emittances above 0.5 at 1.5 µm.
- -
- However, the multiple levels of Co2+ in tetrahedral coordination diminish the selectivity further at high temperatures and higher cobalt content. Low intense electric dipole forbidden transitions show up in emittance and thermal emission spectra at lower energies than the useful emission band as the cobalt concentration increases. Added to that, other radiative transitions involving the first excited state to higher levels may also contribute at high temperatures.
- -
- The microstructural size is not relevant to the thermal emission properties.
- -
- To conclude, as a selective emitter, the cobalt content in the eutectic composite must be kept to a low level in order to avoid selectivity loss. With this limitation, the peak emissivity a 1700 nm remains at 0.6.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Tetrahedrally and Octahedrally Coordinated Co2+ Ions in Mg1-yCoyAl2O4 Spinel
References
- Coutts, T.J. A review of progress in thermophotovoltaic generation of electricity. Renew. Sustain. Energy Rev. 1999, 3, 77–184. [Google Scholar] [CrossRef]
- Licciulli, A.; Diso, D.; Torsello, G.; Tundo, S.; Maffezzoli, A.; Lomascolo, M.; Mazzer, M. The challenge of high-performance selective emitters for thermophotovoltaic applications. Semicond. Sci. Technol. 2003, 18, S174–S183. [Google Scholar] [CrossRef]
- Guazzoni, G.E. High-Temperature Spectral Emittance of Oxides of Erbium, Samarium, Neodymium and Ytterbium. Appl. Spectr. 1972, 26, 60–65. [Google Scholar] [CrossRef]
- Diso, D.; Licciulli, A.; Bianco, A.; Leo, G.; Torsello, G.; Tundo, S.; De Risi, A.; Mazzer, M. Selective emitters for high efficiency TPV conversion: Materials preparation and characterisation. In AIP Conference Proceedings; American Institute of Physics: Melville, NY, USA, 2003; Volume 653, pp. 132–141. [Google Scholar]
- Chubb, D.L.; Pal, A.M.T.; Patton, M.O.; Jenkins, P.P. Rare Earth Doped High Temperature Ceramic Selective Emitters. J. Eur. Ceram. Soc. 1999, 19, 2551–2562. [Google Scholar] [CrossRef] [Green Version]
- Ghanashyam Krishna, M.; Rajendran, M.; Pyke, D.R.; Bhattacharya, A.K. Spectral emissivity of ytterbium oxide-based materials for application as selective emitters in thermophotovoltaic devices. Energy Mater. Sol. Cells 1999, 59, 337–348. [Google Scholar] [CrossRef]
- Nakagawa, N.; Ohtsubo, H.; Waku, Y.; Yugami, H. Thermal emission properties of Al2O3/Er3Al5O12 eutectic ceramics. J. Eur. Ceram. Soc. 2005, 25, 1285–1291. [Google Scholar] [CrossRef]
- Mesa, M.C.; Oliete, P.B.; Merino, R.I.; Orera, V.M. Optical absorption and selective thermal emission in directionally solidified Al2O3-Er3Al5O12 and Al2O3-Er3Al5O12-ZrO2 eutectics. J. Eur. Ceram. 2013, 33, 2587–2596. [Google Scholar] [CrossRef]
- Oliete, P.B.; Mesa, M.C.; Merino, R.I.; Orera, V.M. Directionally solidified Al2O3-Yb3Al5O12 eutectics for selective emitters. Sol. Energy Mater. Sol. Cells 2016, 144, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Oliete, P.B.; Orera, A.; Sanjuán, M.L.; Merino, R.I. Selective thermal emission of directionally solidified Al2O3/Y3−xErxAl5O12 eutectics: Influence of the microstructure, temperature and erbium content. Sol. Energy Mater. Sol. Cells 2018, 174, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Llorca, J.; Orera, V.M. Directionally-solidified eutectic ceramic oxides. Prog. Mater. Sci. 2006, 51, 711–809. [Google Scholar] [CrossRef]
- Ferguson, L.; Fraas, L. Matched Infrared emitters for use with GaSb TPV cells. AIP Conf. Proc. 1997, 401, 169–179. [Google Scholar] [CrossRef]
- Ferguson, L.G.; Dogan, F. A highly efficient NiO-Doped MgO matched emitter for thermophotovoltaic energy conversion. Mater. Sci. Eng. B 2001, 83, 35–41. [Google Scholar] [CrossRef]
- Ferguson, L.G.; Dogan, F. Spectral analysis of transition metal-doped MgO “matched emitters” for thermophotovoltaic energy conversion. J. Mater. Sci. 2002, 37, 1301–1308. [Google Scholar] [CrossRef]
- Sola, D.; Oliete, P.B.; Merino, R.I.; Peña, J.I. Directionally solidified Ni-doped MgO-MgSZ eutectic composites for thermophotovoltaic devices. J. Eur. Ceram. Soc. 2019, 39, 1206–1213. [Google Scholar] [CrossRef]
- Su, S.; Liu, Q.; Hu, Z.; Chen, X.; Pan, H.; Liu, X.; Wu, L.; Li, J. A simple way to prepare Co:MgAl2O4 transparent ceramics for saturable absorber. J. Alloys Compd. 2019, 797, 1288–1294. [Google Scholar] [CrossRef]
- Pappalardo, R.; Wood, D.I.; Linares, R.C., Jr. Optical absorption study of Co-doped oxide systems II. J. Chem. Phys. 1961, 35, 2041–2059. [Google Scholar] [CrossRef]
- Moshtaghioun, B.M.; Peña, J.I.; Merino, R.I. Medium infrared transparency of MgO-MgAl2O4 directionally solidified eutectics. J. Eur. Ceram. Soc. 2020, 40, 1703–1708. [Google Scholar] [CrossRef]
- Tropf, W.J.; Thomas, M.E.; Harris, T.J. Chapter 33: Properties of Crystals end Glasses. In Handbook of Optics. Vol. 2, Devices Measurements and Properties, 2nd ed.; Bass, M., Ed.; Mc. Graw-Hill, Inc.: New York, NY, USA, 1995. [Google Scholar]
- Lushchik, A.; Feldbach, E.; Kotomin, E.A.; Kudryavtseva, I.; Kuzovkov, V.N.; Popov, A.I.; Seeman, V.; Shablonin, E. Distinctive features of diffusion-controlled radiation defect recombination in stoichiometric magnesium aluminate spinel single crystals and transparent polycrystalline ceramics. Sci. Rep. 2020, 10, 7810. [Google Scholar] [CrossRef]
- Kennard, F.L.; Bradt, R.C.; Stubican, V.S. Eutectic solidification of MgO-MgAl2O4. J. Am. Ceram. Soc. 1973, 56, 566–569. [Google Scholar] [CrossRef]
- Moshtaghioun, B.M.; Peña, J.I. Non-Hall-Petch hardness dependence in ultrafine fibrous MgO-MgAl2O4 eutectic ceramics fabricated by the laser-heated flating zome (LFZ) method. J. Eur. Ceram. Soc. 2019, 39, 3208–3212. [Google Scholar] [CrossRef]
- Jackson, K.A.; Hunt, J.D. Lamellar and rod eutetic growth. Trans. Metall. SoC AIME 1966, 236, 1129–1142. [Google Scholar]
- Orkinski, K.; Romaniec, M.; Malinowska, A.; Diduszko, R. Growth-microstructure relationship in MgO-MgAl2O4 eutectic fabricated by micro-pulling down method with MgAl2O4 seed crystals. J. Eur. Ceram. Soc. 2019, 39, 3843–3847. [Google Scholar] [CrossRef]
- Goldstein, A.; Loiko, P.; Burshtein, Z.; Skoptsov, N.; Glazunov, I.; Galun, E.; Kuleshov, N.; Yumashev, K. Development of saturable absorbers for laser passive Q-switching near 1.5 mm based on transparent ceramic Co2+: MgAl2O4. J. Am. Ceram. Soc. 2016, 99, 1324–1331. [Google Scholar] [CrossRef]
- Becker, K.D.; Rau, F. A high-temperature study of defect-induced optical absorption in nickel oxide, Ni1-δO. Ber. Bunsenges. Phys. Chem. 1992, 96, 1017–1027. [Google Scholar] [CrossRef]
- Bosi, F.; Hålenius, U.; D’Hippolito, V.; Andreozzi, G.B. Blue spinel crystals in the MgAl2O4-CoAl2O4 series: Part II. Cation ordering over short-range and long-range scales. Am. Mineral. 2012, 97, 1834–1840. [Google Scholar] [CrossRef]
- Wajler, A.; Kozlowska, A.; Nakielska, M.; Lesniewska-Matys, K.; Sidorowicz, A.; Podniesinski, D.; Putyra, P. Nonlinear absorption of submicrometer grainsize cobalt-doped magnesium aluminates transparent ceramics. J. Am. Ceram. Soc. 2014, 97, 1692–1695. [Google Scholar] [CrossRef]
- Howell, J.R.; Siegel, R.; Mengüç, M.P. Thermal Radiation Heat Transfer, 5th ed.; CRC Press, Taylor and Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Henderson, B.; Imbush, G.G. Optical Spectroscopy of Inorganic Solids; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Redfern, S.A.T.; Harrison, R.J.; O’Neill, H.S.C.; Wood, D.R.R. Thermodynamics and kinetics of cation ordering in MgAl2O4 spinel up to 1600 °C. Am. Mineral. 1999, 84, 299–310. [Google Scholar] [CrossRef]
- He, T.; Becker, K.D. An optical in-situ study of a reacting spinel crystal. Solid State Ion. 1997, 101, 337–342. [Google Scholar] [CrossRef]
- O’Neill, H.S.C. Temperature dependence of the cation distribution in CoAl2O4 spinel. Eur. J. Mineral. 1994, 6, 603–609. [Google Scholar] [CrossRef]











| Sample | Nominal Composition | %at Co Sample | %at Co MgO | %at Co MgAl2O4 | Co2+/cm3 MgO | Co2+/cm3 MgAl2O4 |
|---|---|---|---|---|---|---|
| Co0.04-750 | 67.32MgO-32.53Al2O3-0.15Co3O4 | 0.04 ± 0.02 | 0.14 ± 0.02 | 0.03 ± 0.02 | 1.5 × 1020 | 2.7 × 1019 |
| Co0.04-50 | 0.04 ± 0.02 | 0.13 ± 0.02 | 0.03 ± 0.02 | 1.4 × 1020 | 2.7 × 1019 | |
| Co0.12-50 | 0.12 ± 0.02 | 0.36 ± 0.02 | 0.08 ± 0.02 | 3.9 × 1020 | 8.6 × 1019 | |
| Co0.15-750 | 0.15 ± 0.02 | 0.53 ± 0.025 | 0.07 ± 0.02 | 5.7 × 1020 | 7.6 × 1019 | |
| Co0.22-50 | 67.09MgO-32.41Al2O3-0.5Co3O4 | 0.22 ± 0.02 | 0.69 ± 0.03 | 0.12 ± 0.02 | 7.4 × 1020 | 1.3 × 1020 |
| Co0.29-750 | 0.29 ± 0.02 | 1.02 ± 0.03 | 0.16 ± 0.02 | 1.1 × 1021 | 1.7 × 1020 | |
| Co0.48-750 | 0.48 ± 0.02 | 1.69 ± 0.03 | 0.26 ± 0.02 | 1.8 × 1021 | 2.8 × 1020 | |
| Spinel0.06-50 | 49.89MgO-49.89Al2O3-0.22Co3O4 | 0.06 ± 0.01 | - | 0.06 ± 0.01 | - | 5.9 × 1019 |
| Spinel0.17-50 | 49.62MgO-49.62Al2O3-0.76Co3O4 | 0.17 ± 0.01 | - | 0.17 ± 0.01 | - | 1.8 × 1020 |
| Spinel0.29-50 | 48.75MgO-48.75Al2O3-2.5Co3O4 | 0.29 ± 0.014 | - | 0.29 ± 0.014 | - | 3.0 × 1020 |
| MgO:Co | - | - | 0.14 ± 0.01 | - | 1.5 × 1020 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merino, R.I.; Oliete, P.B.; Moshtaghioun, B.M.; Sola, D.; Peña, J.I. Directionally Solidified Cobalt-Doped MgO-MgAl2O4 Eutectic Composites for Selective Emitters. Appl. Sci. 2022, 12, 10254. https://doi.org/10.3390/app122010254
Merino RI, Oliete PB, Moshtaghioun BM, Sola D, Peña JI. Directionally Solidified Cobalt-Doped MgO-MgAl2O4 Eutectic Composites for Selective Emitters. Applied Sciences. 2022; 12(20):10254. https://doi.org/10.3390/app122010254
Chicago/Turabian StyleMerino, Rosa I., Patricia B. Oliete, Bibi Malmal Moshtaghioun, Daniel Sola, and José I. Peña. 2022. "Directionally Solidified Cobalt-Doped MgO-MgAl2O4 Eutectic Composites for Selective Emitters" Applied Sciences 12, no. 20: 10254. https://doi.org/10.3390/app122010254
APA StyleMerino, R. I., Oliete, P. B., Moshtaghioun, B. M., Sola, D., & Peña, J. I. (2022). Directionally Solidified Cobalt-Doped MgO-MgAl2O4 Eutectic Composites for Selective Emitters. Applied Sciences, 12(20), 10254. https://doi.org/10.3390/app122010254