Quantifying Total Phosphorus and Heavy Metals in Residential Septage
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area, Site Selection, and Tank Pumping
2.2. Septage Characterization and Sampling
2.3. Data and Statistical Analysis
3. Results and Discussion
3.1. Physical and Chemical Characteristics of Septage
3.2. Total Phosphorus and Metal in Septage
3.2.1. Total Phosphorus Concentrations and Masses
3.2.2. Metal Concentrations and Masses
3.3. Mass and Watershed Mass of Pollutants from Domestic Septage
3.4. Land Application of Septage and Potential Environmental Impacts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US EPA. Septic Systems Overview. 2020. Available online: https://www.epa.gov/septic/septic-systems-overview (accessed on 16 February 2022).
- Lusk, M.G.; Toor, G.S.; Yang, Y.; Mechtensimer, S.; De, M.; Obreza, T.A. A review of the fate and transport of nitrogen, phosphorus, pathogens, and trace organic chemicals in septic systems. Crit. Rev. Environ. Sci. Technol. 2017, 47, 455–541. [Google Scholar] [CrossRef]
- US EPA. Onsite Wastewater Treatment Systems Manual; United States Environmental Protection Agency: Washington, DC, USA, 2002.
- Noss, R.R.; Billa, M. Septic System Maintenance Management. J. Urban Plan. Dev. 1988, 114, 73–90. [Google Scholar] [CrossRef]
- Withers, P.J.; Jordan, P.; May, L.; Jarvie, H.P.; Deal, N.E. Do septic tank systems pose a hidden threat to water quality? Front. Ecol. Environ. 2014, 12, 123–130. [Google Scholar] [CrossRef]
- US EPA. Draft EPA Guidelines for Management of Onsite/Decentralized Wastewater Systems; Report # EPA-832-F-00-012; United States Environmental Protection Agency: Washington, DC, USA, 2000.
- Beal, C.D.; Gardner, E.A.; Menzies, N.W. Process, performance, and pollution potential: A review of septic tank-soil absorption systems. Aust. J. Soil Res. 2005, 43, 781. [Google Scholar] [CrossRef] [Green Version]
- Efroymson, R.A.; Jones, D.S.; Gold, A.J. An Ecological Risk Assessment Framework for Effects of Onsite Wastewater Treatment Systems and Other Localized Sources of Nutrients on Aquatic Ecosystems. Hum. Ecol. Risk Assess. 2007, 13, 574–614. [Google Scholar] [CrossRef]
- Humphrey, C.; Jernigan, J.; Iverson, G.; Serozi, B.; O’Driscoll, M.; Pradhan, S.; Bean, E. Field Evaluation of Nitrogen Treatment by Conventional and Single-Pass Sand Filter Onsite Wastewater Systems in the North Carolina Piedmont. Water Air Soil Pollut. 2016, 227, 255. [Google Scholar] [CrossRef]
- Humphrey, C.; Serozi, B.; Iverson, G.; Jernigan, J.; Pradhan, S.; O’Driscoll, M.; Bean, E. Phosphate treatment by onsite wastewater systems in nutrient-sensitive watersheds of North Carolina’s Piedmont. Water Sci. Technol. 2016, 74, 1527–1538. [Google Scholar] [CrossRef]
- US EPA. Domestic Septage Regulatory Guidance; Report # EPA 832-B-92-005; United States Environmental Protection Agency: Washington, DC, USA, 1993.
- US EPA. Biosolids Technology Fact Sheet: Land Application of Biosolids; Report # EPA 832-F-00-064; United States Environmental Protection Agency: Washington, DC, USA, 2000.
- US EPA. Land Application of Sewage Sludge; Report # EPA/831-B-93-002b; United States Environmental Protection Agency: Washington, DC, USA, 1994.
- Wang, H.; Brown, S.; Magesan, G.; Slade, A.; Quintern, M.; Clinton, P.; Payn, T. Technological options for the management of biosolids. Environ. Sci. Pollut. Res. 2008, 15, 308–317. [Google Scholar] [CrossRef]
- Singh, R.P.; Agrawal, M. Potential benefits and risks of land application of sewage sludge. Waste Manag. 2008, 28, 347–358. [Google Scholar] [CrossRef]
- Fodor, L.; Szabó, L. Study of Heavy Metal Leaching in the Soil. In Proceedings of the 13th International Soil Conversation Organization Conference, Conserving Soil and Water for Society: Sharing Solutions, Brisbane, Australia, 4–8 July 2004; p. 4. [Google Scholar]
- Mata-González, R.; Sosebee, R.E.; Wan, C. Shoot and root biomass of desert grasses as affected by biosolids application. J. Arid Environ. 2002, 50, 477–488. [Google Scholar] [CrossRef]
- Stöven, K.; Schnug, E. Long term effects of heavy metal enriched sewage sludge disposal in agriculture on soil biota. Agri. For. Res. 2009, 59, 131–138. [Google Scholar]
- Sloan, J.J.; Dowdy, R.H.; Dolan, M.S. Recovery of Biosolids-Applied Heavy Metals Sixteen Years after Application. J. Environ. Qual. 1998, 27, 1312–1317. [Google Scholar] [CrossRef]
- Milinovic, J.; Vidal, M.; Lacorte, S.; Rigol, A. Leaching of heavy metals and alkylphenolic compounds from fresh and dried sewage sludge. Environ. Sci. Pollut. Res. 2014, 21, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Wierzbowska, J.; Sienkiewicz, S.; Krzebietke, S.; Bowszys, T. Heavy Metals in Water Percolating Through Soil Fertilized with Biodegradable Waste Materials. Water Air Soil Pollut. 2016, 227, 456. [Google Scholar] [CrossRef] [Green Version]
- McLaren, R.G.; Clucas, L.M.; Taylor, D.M. Leaching of macronutrients and metals from undisturbed soils treated with metal-spiked sewage sludge. 3. Distribution of residual metals. Aust. J. Soil Res. 2005, 43, 159–170. [Google Scholar] [CrossRef]
- Heckrath, G.; Brookes, P.C.; Poulton, P.R.; Goulding, K.W.T. Phosphorus leaching from soils containing different phosphorus concentrations in the Broadbalk Experiment. J. Environ. Qual. 1995, 24, 904–910. [Google Scholar] [CrossRef]
- Humphrey, C.P.; O’Driscoll, M.A. Biogeochemistry of groundwater beneath onsite wastewater systems in a coastal watershed. Univer. J. Environ. Res. Technol. 2011, 1, 320–328. [Google Scholar]
- Humphrey, C.P.; O’Driscoll, M.A.; Mallinson, D.; Hardison, S. Geophysical and Water Quality Characterization of On-Site Wastewater Plumes; Report # UNC-WRRI-445; The University of North Carolina: Raleigh, NC, USA, 2013. [Google Scholar]
- Humphrey, C.; O’Driscoll, M.; Deal, N.; Lindbo, D. Fate and Transport of Phosphate from an Onsite Wastewater System in Beaufort County, North Carolina. J. Environ. Health 2014, 76, 28–33. [Google Scholar]
- Humphrey, C.; Pradhan, S.; Bean, E.; O’Driscoll, M.; Iverson, G. Preliminary Evaluation of a Permeable Reactive Barrier for Reducing Groundwater Nitrate Transport from a Large Onsite Wastewater System. Am. J. Environ. Sci. 2015, 11, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Gold, A.J.; Sims, J.T. Research needs in decentralized wastewater treatment and management: A risk-based approach to nutrient contamination. In Proceedings of the National Research Needs Conference Proceedings: Risk-Based Decision Making for Onsite Wastewater Treatment, St. Louis, MO, USA, 19–20 May 2000; pp. 1–37. [Google Scholar]
- Lowe, K.S.; Tucholke, M.B.; Tomaras, J.M.B.; Conn, K.; Hoppe, C.; Drewes, J.E.; McCray, J.E.; Munakata-Marr, J. Influent Constituent Characteristics of the Modern Waste Stream from Single Sources: Literature Review; Water Environment Research Foundation: London, UK, 2007. [Google Scholar]
- Wilhelm, S.R.; Schiff, S.L.; Robertson, W.D. Chemical fate and transport in a domestic septic system: Unsaturated and saturated zone geochemistry. Environ. Toxicol. Chem. 1994, 13, 193–203. [Google Scholar] [CrossRef]
- Humphrey, C.; Anderson-Evans, E.; O’Driscoll, M.; Manda, A.; Iverson, G. Comparison of Phosphorus Concentrations in Coastal Plain Watersheds Served by Onsite Wastewater Treatment Systems and a Municipal Sewer Treatment System. Water Air Soil Pollut. 2015, 226, 2259. [Google Scholar] [CrossRef]
- Hoover, M.T.; Disy, T.A.; Pfieffer, M.A.; Dudley, N.; Mayer, R.B.; Buffington, B. North Carolina Subsurface Wastewater Operators Training School Manual, 1st ed.; Department of Environment, Health and Natural Resources, North Carolina State University: Raleigh, NC, USA, 1996; pp. 4.1–4.31. [Google Scholar]
- Pradhan, S.S.; Hoover, M.T.; Austin, R.E.; Devine, H.A. Potential Nitrogen Contributions from On-Site Wastewater Treatment Systems to North Carolina’s River Basins and Sub-Basins; Technical Bulletin 324; NC State University: Raleigh, NC, USA, 2007. [Google Scholar]
- Sauer, P.A.; Tyler, E.J. Heavy metal and volatile organic chemical removal and treatment in on-site wastewater infiltration systems. I. Catch basins and septic tanks. Water Air Soil Pollut. 1996, 89, 221–232. [Google Scholar] [CrossRef]
- Brandes, M. Accumulation rate and characteristics of septic tank sludge and septage. J. Water Pollut. Control Fed. 1978, 50, 936–943. [Google Scholar]
- City of Durham. Falls Lake Watershed. 2021. Available online: https://durhamnc.gov/716/Falls-Lake (accessed on 16 February 2022).
- UNRBA. Lick Creek Watershed Restoration Plan; North Carolina Division of Water Quality: Raleigh, NC, USA, 2009.
- NC DEQ. Integrated Report Files for NC 303(d) and 305(b) Files. 2021. Available online: https://deq.nc.gov/about/divisions/water-resources/planning/modeling-assessment/water-quality-data-assessment/integrated-report-files (accessed on 16 February 2022).
- US EPA. Method 200.7 Trace Elements in Water, Solids, and Biosolids by Inductively Coupled Plasma-Atomic Spectrometry, Revision 5.0; Report # EPA-821-R-01-010; United States Environmental Protection Agency: Washington, DC, USA, 2001.
- Donohue, S.J.; Aho, D.W. Determination of P, K, Ca, Mg, Mn, Fe, Al, B, Cu, and Zn in plant tissue by inductively coupled plasma (ICP) emission spectroscopy. In Plant Analysis Reference Procedures for the Southern Region of the United States; Plank, C.O., Ed.; Southern Cooperative Series Bulletin 368; Georgia Cooperative Extension Service: Athens, GA, USA, 1992; pp. 34–37. [Google Scholar]
- Campbell, C.R.; Plank, C.O. Sample preparation. In Plant Analysis Reference Procedures for the Southern Region of the United States; Plank, C.O., Ed.; Southern Cooperative Series Bulletin 368; Georgia Cooperative Extension Service: Athens, GA, USA, 1992; pp. 1–12. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 6 April 2021).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H.; Bryan, J. Readxl: Read Excel Files. 2019. Available online: https://CRAN.R-project.org/package=readxl (accessed on 6 April 2021).
- Wilke, C.O. Cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. 2019. Available online: https://CRAN.R-project.org/package=cowplot (accessed on 6 April 2021).
- US EPA. How to Care for Your Septic System. 2021. Available online: https://www.epa.gov/septic/how-care-your-septic-system#:~:text=Maintain%20Your%20Drainfield-,Inspect%20and%20Pump%20Frequently,every%20three%20to%20five%20years (accessed on 21 January 2022).
- Mancl, K. Estimating Septic Tank Pumping Frequency. J. Environ. Eng. 1984, 110, 283–285. [Google Scholar] [CrossRef]
- US Census. QuickFacts for Durham County, North Carolina, USA. 2021. Available online: https://www.census.gov/quickfacts/durhamcountynorthcarolina (accessed on 22 January 2022).
- Robertson, W.D. Development of steady-state phosphate concentrations in septic system plumes. J. Contam. Hydrol. 1995, 19, 289–305. [Google Scholar] [CrossRef]
- Robertson, W.D.; Blowes, D.W. Major ion and trace metal geochemistry on an acidic septic-system plume in silt. Groundwater 1995, 33, 275–283. [Google Scholar] [CrossRef]
- McCray, J.E.; Kirkland, S.L.; Siegrist, R.L.; Thyne, G.D. Model Parameters for Simulating Fate and Transport of On-Site Wastewater Nutrients. Groundwater 2005, 43, 628–639. [Google Scholar] [CrossRef]
- Schellenger, F.L.; Hellweger, F.L. Phosphorus loading from onsite wastewater systems to a lake (at long time scales). Lake Reserv. Manag. 2019, 35, 90–101. [Google Scholar] [CrossRef]
- Schroder, J.L.; Zhang, H.; Zhou, D.; Basta, N.; Raun, W.R.; Payton, M.E.; Zazulak, A. The Effect of Long-Term Annual Application of Biosolids on Soil Properties, Phosphorus, and Metals. Soil Sci. Soc. Am. J. 2008, 72, 73–82. [Google Scholar] [CrossRef]
- Barbarick, K.A.; Ippolito, J.A.; Westfall, D.G. Extractable Trace Elements in the Soil Profile after Years of Biosolids Application. J. Environ. Qual. 1998, 27, 801–805. [Google Scholar] [CrossRef]
- Krithika, D.; Thomas, A.R.; Iyer, G.R.; Kranert, M.; Philip, L. Spatio-temporal variation of septage characteristics of a semi-arid metropolitan city in a developing country. Environ. Sci. Pollut. Res. 2017, 24, 7060–7076. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Guide to Septage Treatment and Disposal; Report # EPA/625/R-94/002; United States Environmental Protection Agency: Washington, DC, USA, 1994.
- Lester, J.N. Significance and behaviour of heavy metals in waste water treatment processes I. Sewage treatment and effluent discharge. Sci. Total Environ. 1983, 30, 1–44. [Google Scholar] [CrossRef]
- Goldstone, M.E.; Kirk, P.W.W.; Lester, J.N. The behaviour of heavy metals during wastewater treatment II. Lead, nickel and zinc. Sci. Total Environ. 1990, 95, 253–270. [Google Scholar] [CrossRef]
- Kempton, S.; Sterritt, R.M.; Lester, J.N. Heavy metal removal in primary sedimentation I. The influence of metal solubility. Sci. Total Environ. 1987, 63, 231–246. [Google Scholar] [CrossRef]
- Wang, M.; Chen, C.; Ju, Y.; Tsai, M.; Chen, C.; Dong, C. Distribution and environmental risk assessment of trace metals in sludge from multiple sources in Taiwan. J. Environ. Sci. Health Part A 2021, 56, 481–491. [Google Scholar] [CrossRef]
- Whelan, B.R.; Titamnis, Z.V. Daily chemical variability of domestic septic tank effluent. Water Air Soil Pollut. 1982, 17, 131–139. [Google Scholar] [CrossRef]
- Ptacek, C.J. Geochemistry of a septic-system plume in a coastal barrier bar, Point Pelee, Ontario, Canada. J. Contam. Hydrol. 1998, 33, 293–312. [Google Scholar] [CrossRef]
- Sotirakou, E.; Kladitis, G.; Diamantis, N.; Grigoropoulou, H. Ammonia and Phosphorous removal in municipal wastewater treatment plant with extended aeration. Glob. Nest J. 2013, 1, 47–53. [Google Scholar]
- Chen, K.Y.; Young, C.S.; Jan, T.K.; Rohatgi, N. Trace metals in wastewater effluents. J. Water Pollut. Control Fed. 1974, 46, 2663–2675. [Google Scholar]
- Firfilionis, G.; Paraskevopoulou, V.; Vilioti, G.; Dassenakis, M. The removal of trace metals at the wastewater treatment plant of Psyttalia. Mediterr. Mar. Sci. 2004, 5, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Mapanda, F.; Mangwayana, E.N.; Nyamangara, J.; Giller, K.E. The effect of long-term irrigation using wastewater on heavy metal contents of soils under vegetables in Harare, Zimbabwe. Agric. Ecosyst. Environ. 2005, 107, 151–165. [Google Scholar] [CrossRef]
- Milik, J.; Pasela, R.; Lachowicz, M.; Chalamoński, M. The concentration of trace elements in sewage sludge from wastewater treatment plant in Gniewino. J. Ecol. Eng. 2017, 18, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Healy, M.G.; Barrett, M.; Lanigan, G.J.; João Serrenho, A.; Ibrahim, T.G.; Thornton, S.F.; Rolfe, S.A.; Huang, W.E.; Fenton, O. Optimizing nitrate removal and evaluating pollution swapping trade-offs from laboratory denitrification bioreactors. Ecol. Eng. 2015, 74, 290–301. [Google Scholar] [CrossRef] [Green Version]
- US EPA. Regulatory Determinations for Pollutants in Biosolids. 2022. Available online: https://www.epa.gov/biosolids/regulatory-determinations-pollutants-biosolids (accessed on 7 February 2022).
- Lossing, H.; Champagne, P.; McLellan, P.J. Examination of sludge accumulation rates and sludge characteristics for a decentralized community wastewater treatment systems with individual primary clarifier tanks located in Wardsville (Ontario, Canada). Water Sci. Technol. 2010, 62, 2944–2952. [Google Scholar] [CrossRef] [Green Version]
- Tjandraatmadja, G.; Pollard, C.; Sheedy, C.; Gozukara, Y. Sources of Contaminants in Domestic Wastewater: Nutrients and Additional Elements from Household Products; CSIRO Publishing: Clayton, Australia, 2010. [Google Scholar]
- Lowe, K.S.; Tucholke, M.B.; Tomaras, J.M.B.; Conn, K.; Hoppe, C.; Drewes, J.E.; McCray, J.E.; Munakata-Marr, J. Influent Constituent Characteristics of the Modern Waste Stream from Single Sources, 1st ed.; IWA Publishing: London, UK, 2009. [Google Scholar]
- Crites, R.W.; Tchobanoglous, G. Small and Decentralized Wastewater Management Systems, 1st ed.; McGraw-Hill: Boston, MA, USA, 1998; p. 1104. [Google Scholar]
- Chen, X.; Cheng, X.; Chen, B.; Sun, D.; Ma, W.; Wang, X. Phosphorus precipitation in septic systems induced by iron reduction: A process for phosphorus removal under anaerobic conditions. Desalin. Water Treat. 2015, 54, 2891–2901. [Google Scholar] [CrossRef]
- Robertson, W.D.; Schiff, S.L.; Ptacek, C.J. Review of phosphate mobility and persistence in 10 septic system plumes. Groundwater 1998, 36, 1000–1010. [Google Scholar] [CrossRef]
- Olivares, M.; Uauy, R. Copper as an essential nutrient. Am. J. Clin. Nutr. 1996, 63, 791S–796S. [Google Scholar] [CrossRef]
- Saper, R.B.; Rash, R. Zinc: An Essential Micronutrient. Am. Fam. Physician 2009, 79, 768–772. [Google Scholar]
- Anke, M.; Groppel, B.; Kronemann, H.; Grün, M. Nickel—An essential element. IARC Sci. Publ. 1984, 53, 339–365. [Google Scholar]
- Brown, P.H.; Welch, R.M.; Cary, E.E. Nickel: A Micronutrient Essential for Higher Plants. Plant Physiol. 1987, 85, 801–803. [Google Scholar] [CrossRef]
- Naturvardsverket. Vad Innehaller Avlopp fran Hushall; Report 4425; Swedish Environment Protection Agency: Stockholm, Sweden, 1995. (In Swedish)
- Sörme, L.; Lagerkvist, R. Sources of heavy metals in urban wastewater in Stockholm. Sci. Total Environ. 2002, 298, 131–145. [Google Scholar] [CrossRef]
- US CDC. Lead in Paint. 2022. Available online: https://www.cdc.gov/nceh/lead/prevention/sources/paint.htm (accessed on 8 February 2022).
- US EPA. Use of Lead Free Pipes, Fittings, Fixtures, Solder, and Flux for Drinking Water. 2021. Available online: https://www.epa.gov/sdwa/use-lead-free-pipes-fittings-fixtures-solder-and-flux-drinking-water (accessed on 8 February 2022).
- Iverson, G.; Humphrey, C.P.; O’Driscoll, M.A.; Sanderford, C.; Jernigan, J.; Serozi, B. Nutrient exports from watersheds with varying septic system densities in the North Carolina Piedmont. J. Environ. Manag. 2018, 211, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Nyamukamba, P.; Moloto, M.; Tavengwa, N.; Ejidike, I. Evaluating Physicochemical Parameters, Heavy Metals, and Antibiotics in the Influents and Final Effluents of South African Wastewater Treatment Plants. Pol. J. Environ. Stud. 2019, 28, 1305–1312. [Google Scholar] [CrossRef] [Green Version]
- Mohlander, U. Tungmetaller i Tappvatten; The Environmental Administration: Stockholm, Sweden, 1992. (In Swedish) [Google Scholar]
- Tiruneh, A.T.; Fadiran, A.O.; Mtshali, J.S. Evaluation of the risk of heavy metals in sewage sludge intended for agricultural application in Swaziland. Int. J. Environ. Sci. 2014, 5, 197–216. [Google Scholar]
- O’Driscoll, M.; DeWalle, D.; Humphrey, C.; Iverson, G. Groundwater Seeps: Portholes to Evaluate Groundwater’s Influence on Stream Water Quality. J. Contemp. Water Res. Ed. 2019, 166, 57–78. [Google Scholar] [CrossRef] [Green Version]
- US EPA. Land Application of Sewage Sludge and Domestic Septage; Report # EPA/625/R-95/001; United States Environmental Protection Agency: Washington, DC, USA, 1995.
- NC DEQ. NC Surface Water Classifications. 2022. Available online: http://ncdenr.maps.arcgis.com/apps/webappviewer/index.html?id=6e125ad7628f494694e259c80dd64265 (accessed on 11 February 2022).
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.A.; Johnson, M.V.; Morton, S.L.; Perkins, D.A.K.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef]
- Jadia, C.D.; Fulekar, M.H. Phytoremediation of heavy metals: Recent techniques. Afr. J. Biotechnol. 2010, 8, 921–928. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- Sharpley, A.N.; Weld, J.L.; Beegle, D.B.; Kleinman, P.J.A.; Gburek, W.J.; Moore, P.A.; Mullins, G. Development of phosphorus indices for nutrient management planning strategies in the United States. J. Soil Water Conserv. 2003, 58, 137–152. [Google Scholar]
- Gatiboni, L.; Hardy, D.; Osmond, D. Changes in the Phosphorus Fertilizer Recommendations for Corn, Soybean, and Small Grains in North Carolina. 2021. Available online: https://content.ces.ncsu.edu/changes-in-phosphorus-fertilizer-recommendations-for-corn-soybean-and-small-grains (accessed on 11 February 2022).
- Osmond, D.L.; Cabrera, M.L.; Feagley, S.E.; Hardee, G.E.; Mitchell, C.C.; Moore, P.A.J.; Mylavarapu, R.S.; Oldham, J.L.; Stevens, J.C.; Thom, W.O. Comparing ratings of the southern phosphorus indices. J. Soil Water Conserv. 2006, 61, 325–337. [Google Scholar]
- Osmond, D.; Sharpley, A.; Bolster, C.; Cabrera, M.; Feagley, S.; Lee, B.; Mitchell, C.; Mylavarapu, R.; Oldham, L.; Walker, F.; et al. Comparing Phosphorus Indices from Twelve Southern U.S. States against Monitored Phosphorus Loads from Six Prior Southern Studies. J. Environ. Qual. 2012, 41, 1741–1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodds, W.K.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, K.L.; Riley, A.J.; Schloesser, J.T.; Thornbrugh, D.J. Eutrophication of U.S. Freshwaters: Analysis of Potential Economic Damages. Environ. Sci. Technol. 2009, 43, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, U. Enhancing Phytoextraction: The Effect of Chemical Soil Manipulation on Mobility, Plant Accumulation, and Leaching of Heavy Metals. J. Environ. Qual. 2003, 32, 1939–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Tank Layer | pH | Temperature (°C) | DO (mg L−1) | ORP (mV) | SC (µS cm−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (Range) | Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | Mean (SD) | |
| Scum | 6.79 (3.91–7.40) | 6.45 (1.06) | 27.12 (12.40–32.04) | 24.14 (6.72) | 1.47 (0.45–9.31) | 2.72 (2.90) | −174 (−491–−15) | −184 (154) | 1038 (226–2426) | 1193 (855) |
| Liquid | 6.87 (5.36–8.04) | 6.89 (0.52) | 26.79 (11.36–34.90) | 25.05 (5.83) | 0.85 (0.17–10.07) | 1.75 (2.43) | −296 (−554–14) | −278 (127) | 1380 (129–2742) | 1414 (718) |
| Sludge | 6.69 (5.66–7.77) | 6.68 (0.44) | 25.07 (12.39–32.07) | 23.26 (5.53) | 0.63 (0.17–8.8) | 1.60 (2.37) | −275 (−508–−1) | −262 (121) | 1797 (184–3325) | 1810 (981) |
| Metal | Median (Range) | Mean (SD) | ||||
|---|---|---|---|---|---|---|
| Scum | Liquid | Sludge | Scum | Liquid | Sludge | |
| Cadmium | ||||||
| Conc (mg L−1) | <0.01 (<0.01–0.80) | <0.01 (<0.01–0.30) | <0.01 (<0.01–0.60) | 0.09 (0.27) | 0.03 (0.08) | 0.07 (0.20) |
| Mass (g) | <0.01 (<0.01–3.03) | <0.01 (<0.01–1.14) | <0.01 (<0.01–2.27) | 0.14 (0.66) | 0.11 (0.32) | 0.24 (0.72) |
| Copper | ||||||
| Conc (mg L−1) | 1.15 (0.06–206.00) | 0.40 (0.04–4.80) | 0.60 (0.05–222.00) | 18.21 (56.53) | 0.85 (1.28) | 11.63 (43.87) |
| Mass (g) | 0.23 (<0.01–780.22) | 1.51 (0.15–18.18) | 1.89 (<0.01–840.82) | 35.86 (155.43) | 3.24 (4.85) | 41.39 (161.22) |
| Lead | ||||||
| Conc (mg L−1) | 0.60 (<0.01–24.50) | 0.50 (<0.01–4.30) | 0.40 (<0.01–12.50) | 3.19 (8.00) | 0.79 (1.17) | 1.61 (3.51) |
| Mass (g) | <0.01 (<0.01–92.79) | 1.89 (<0.01–16.29) | 1.51 (<0.01–47.34) | 5.18 (20.11) | 2.99 (4.43) | 5.44 (12.66) |
| Nickel | ||||||
| Conc (mg L−1) | 0.20 (<0.01–3.70) | 0.20 (<0.01–1.50) | 0.20 (<0.01–4.00) | 0.79 (1.27) | 0.31 (0.39) | 0.52 (0.94) |
| Mass (g) | <0.01 (<0.01–14.01) | 0.76 (<0.01–5.68) | 0.76 (<0.01–15.15) | 1.28 (3.40) | 1.16 (1.46) | 1.77 (3.43) |
| Zinc | ||||||
| Conc (mg L−1) | 3.00 (0.60–358.00) | 7.92 (<0.01–76.80) | 2.14 (0.30–256.00) | 33.90 (97.65) | 13.50 (16.86) | 18.62 (55.88) |
| Mass (g) | 2.27 (<0.01–1355.92) | 30.00 (<0.01–290.88) | 7.76 (<0.01–969.60) | 66.76 (269.60) | 51.15 (63.87) | 66.25 (205.64) |
| Source | Total Phosphorus | Cadmium | Copper | |||
| Conc (mg L−1) | Mass (g) | Conc (mg L−1) | Mass (g) | Conc (mg L−1) | Mass (g) | |
| Septic, liquid 1 | 1.20–39.5 | 4.54–149.61 a | <0.01–0.04 | <0.03–0.15 b | 0.01–0.12 | 0.03–0.45 b |
| Septic, sludge 2 | 18–610 | 61.36–2079.33 a | 0.06–0.35 | 0.23–1.33 a | - | - |
| Sewer, liquid 3 | 4–180 | - | 0.56–11 | - | 52.2–102 | - |
| Sewer, sludge 4 | 8000–59,500 | - | 0.49–30 | - | 0.66–1160 | - |
| Septage, composite 5 | 5–760 | 75.75–2879 a | 0.005–11 | 0.07–42 a | 0.01–640 | 2.18–2424 a |
| Ceiling conc 6 | 85 | 39 | 4300 | 1500 | ||
| Source | Lead | Nickel | Zinc | |||
| Conc (mg L−1) | Mass (g) | Conc (mg L−1) | Mass (g) | Conc (mg L−1) | Mass (g) | |
| Septic, liquid 1 | 0.01–0.19 | 0.04–0.72 a | <0.02 | <0.06 | 0.04–0.18 | 0.12–0.68 b |
| Septic, sludge 2 | 1.01–7.70 | 3.83–29.16 a | - | - | - | - |
| Sewer, liquid 3 | 28.6–98 | - | 32.2–191 | - | 0.46–182 | - |
| Sewer, sludge 4 | 2.72–1600 | - | 3.37–177 | - | 79–1980 | - |
| Septage, composite 5 | <0.025–791 | <0.09–2996 a | 0.01–37 | 0.04–140.14 a | <0.01–1273 | <0.01–4821 a |
| Ceiling conc 6 | 840 | 300 | 420 | 420 | 7500 | 2800 |
| Pollutant | Median (Range) | Mean (SD) | ||
|---|---|---|---|---|
| Weighted Mass (g) | Watershed a Mass (g) | Weighted Mass (g) | Watershed a Mass (g) | |
| TP | 19.5 (0.9–1041) | 1694 (76.3–90587) | 66.6 (178) | 5792 (15,483) |
| Cadmium | <0.01 (<0.01–2.0) | <0.9 (<0.9–173) | 0.2 (0.5) | 15.3 (45.7) |
| Copper | 1.3 (0.1–520) | 117 (4.7–45,238) | 19.3 (86.9) | 1681 (7562) |
| Lead | 1.8 (<0.01–44.2) | 152 (<0.9–3847) | 4.8 (10.8) | 419 (937) |
| Nickel | 1.3 (<0.01–4.3) | 112 (<0.9–378) | 1.5 (1.4) | 129 (121) |
| Zinc | 13.8 (0.3–788) | 1202 (26.8–68,514) | 55.6 (136) | 4838 (11,865) |
| Tank | Layer | Layer Thickness | Pollutant Concentration (mg L−1) | |||||
|---|---|---|---|---|---|---|---|---|
| (%) | TP | Cadmium | Copper | Lead | Nickel | Zinc | ||
| 1 | Scum | 0% | ||||||
| 2 | Scum | 19% | 835 | 0.8 | 206 | 24.5 | 2.1 | 358 |
| 3 | Scum | 4% | 54.4 | 0 | 9 | 0 | 0 | 1.4 |
| 4 | Scum | 0% | ||||||
| 1 | Liquid | 84% | 8.5 | 0.2 | 4.5 | 3.3 | 0.1 | 60 |
| 2 | Liquid | 38% | 21.6 | 0.3 | 4.1 | 4.3 | 0.3 | 76.8 |
| 3 | Liquid | 72% | 8.2 | 0 | 4.8 | 0 | 0 | 0.8 |
| 4 | Liquid | 72% | 9.8 | 0 | 1.6 | 0 | 0.2 | 3.8 |
| 1 | Sludge | 16% | 199 | 0.6 | 112 | 9 | 4 | 192 |
| 2 | Sludge | 44% | 252 | 0.6 | 222 | 12.5 | 1.1 | 256 |
| 3 | Sludge | 24% | 3.2 | 0 | 8.2 | 0 | 0 | 1.1 |
| 4 | Sludge | 28% | 45.7 | 0 | 2.8 | 0.3 | 0.1 | 2.8 |
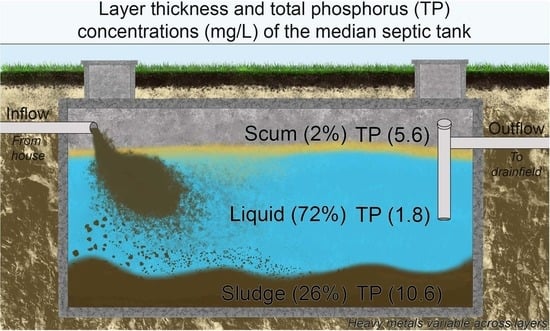
| Median (Range) | Scum | 2% (0–19%) | 444 (54.4–835) | 0.4 (<0.01–0.8) | 108 (9–206) | 12.3 (<0.01–24.5) | 1.1 (<0.01–2.1) | 180 (1.4–358) |
| Liquid | 72% (38–84%) | 9.2 (8.2–21.6) | 0.1 (<0.01–0.3) | 4.3 (1.6–4.8) | 1.7 (<0.01–4.3) | 0.2 (<0.01–0.3) | 31.9 (0.8–76.8) | |
| Sludge | 26% (16–44%) | 122 (3.2–252) | 0.3 (<0.01–0.6) | 60.1 (2.8–222) | 4.7 (<0.01–12.5) | 0.6 (<0.01–4.0) | 97.4 (1.1–256) | |
| Mean (SD) | Scum | 6% (9%) | 444 (552) | 0.4 (0.6) | 108 (139) | 12.3 (17.3) | 1.1 (1.5) | 180 (252) |
| Liquid | 66% (20%) | 12.0 (6.4) | 0.1 (0.2) | 3.8 (1.5) | 1.9 (2.2) | 0.2 (0.1) | 35.4 (38.8) | |
| Sludge | 28% (12%) | 125 (119) | 0.3 (0.3) | 86.3 (104) | 5.5 (6.3) | 1.3 (1.9) | 113 (131) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iverson, G.; Humphrey, C.P., Jr.; O’Driscoll, M.; Jernigan, J.; Serozi, B.; Sanderford, C. Quantifying Total Phosphorus and Heavy Metals in Residential Septage. Appl. Sci. 2022, 12, 3336. https://doi.org/10.3390/app12073336
Iverson G, Humphrey CP Jr., O’Driscoll M, Jernigan J, Serozi B, Sanderford C. Quantifying Total Phosphorus and Heavy Metals in Residential Septage. Applied Sciences. 2022; 12(7):3336. https://doi.org/10.3390/app12073336
Chicago/Turabian StyleIverson, Guy, Charles P. Humphrey, Jr., Michael O’Driscoll, Jordan Jernigan, Brent Serozi, and Christa Sanderford. 2022. "Quantifying Total Phosphorus and Heavy Metals in Residential Septage" Applied Sciences 12, no. 7: 3336. https://doi.org/10.3390/app12073336
APA StyleIverson, G., Humphrey, C. P., Jr., O’Driscoll, M., Jernigan, J., Serozi, B., & Sanderford, C. (2022). Quantifying Total Phosphorus and Heavy Metals in Residential Septage. Applied Sciences, 12(7), 3336. https://doi.org/10.3390/app12073336