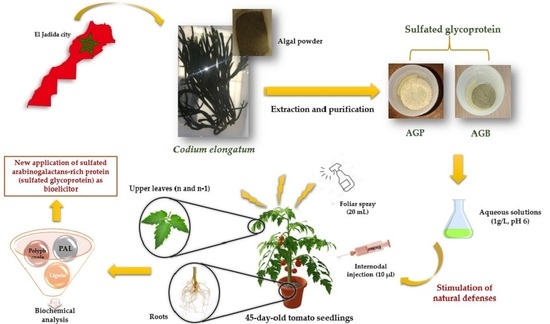
A Novel Sulfated Glycoprotein Elicitor Extracted from the Moroccan Green Seaweed Codium decorticatum Induces Natural Defenses in Tomato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of C. decorticatum Samples
2.2. Extraction and Purification of Sulfated Polysaccharides from C. decorticatum
2.3. Chemical Composition
2.4. HPSEC-MALLS Analysis
2.5. Monosaccharide Compositions Analysis by GC-EI/MS
2.6. ATR-FTIR Spectroscopy
2.7. Elicitor Applications
2.8. Phenylalanine Ammonia Lyase (PAL) Activity and Protein Assays
2.9. Extraction and Purification of Total Phenolic Compounds
2.10. Extraction and Quantification of Lignin Content
2.11. Statistical Analysis
3. Results
3.1. Extraction Yield and Chemical Composition of AGB and AGP Fractions
3.2. Molecular Weight Analysis of AGB and AGP Polysaccharides by SEC-MALLS
3.3. Monosaccharide Compositions of AGB and AGP Fractions
3.4. Fourier Transform Infrared Spectroscopy
3.5. Effect of AGB and AGP Fractions on the Induction of PAL Activity in the Roots and the Leaves of Tomato Seedlings
3.6. Effect of AGB and AGP Fractions on the Accumulation of Phenolic Compounds in the Roots and the Leave Tissues of Tomato Seedlings
3.7. Effect of AGB and AGP Fractions on the Lignin Content in the Root and the Leaf Tissues of Tomato Seedlings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Bioactive Components from Seaweeds. In Sea Plants; Academic Press: Cambridge, MA, USA, 2014; pp. 345–378. [Google Scholar]
- Rioux, L.-E.; Turgeon, S.L. Seaweed carbohydrates. In Seaweed Sustainability; Academic Press: Cambridge, MA, USA, 2015; pp. 141–192. [Google Scholar]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Modafar, C.; Elgadda, M.; El Boutachfaiti, R.; Abouraicha, E.; Zehhar, N.; Petit, E.; El Alaoui-Talibi, Z.; Courtois, B.; Courtois, J. Induction of natural defence accompanied by salicylic acid-dependant systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Sci. Hortic. 2012, 138, 55–63. [Google Scholar] [CrossRef]
- Farias, E.H.; Pomin, V.H.; Valente, A.P.; Nader, H.B.; Rocha, H.A.; Mourao, P.A. A preponderantly 4-sulfated, 3-linked galactan from the green alga Codium isthmocladum. Glycobiology 2008, 18, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Bouissil, S.; Alaoui-Talibi, Z.E.; Pierre, G.; Rchid, H.; Michaud, P.; Delattre, C.; El Modafar, C. Fucoidans of Moroccan Brown Seaweed as Elicitors of Natural Defenses in Date Palm Roots. Mar. Drugs 2020, 18, 596. [Google Scholar] [CrossRef] [PubMed]
- Abouraïcha, E.; El Alaoui-Talibi, Z.; El Boutachfaiti, R.; Petit, E.; Courtois, B.; Courtois, J.; El Modafar, C. Induction of natural defense and protection against Penicillium expansum and Botrytis cinerea in apple fruit in response to bioelicitors isolated from green algae. Sci. Hortic. 2015, 181, 121–128. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A.J.B. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Fernández, P.V.; Arata, P.X.; Ciancia, M. Polysaccharides from Codium Species. In Sea Plants; Academic Press: Cambridge, MA, USA, 2014; pp. 253–278. [Google Scholar]
- Provan, J.; Murphy, S.; Maggs, C.A. Tracking the invasive history of the green alga Codium fragile ssp. tomentosoides. Mol. Ecol. 2005, 14, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Wu, H.; Liu, R. Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar. Drugs 2014, 12, 4984–5020. [Google Scholar] [CrossRef] [PubMed]
- Fernández, P.V.; Estevez, J.M.; Cerezo, A.S.; Ciancia, M. Sulfated β-d-mannan from green seaweed Codium vermilara. Carbohydr. Polym. 2012, 87, 916–919. [Google Scholar] [CrossRef]
- Fernandez, P.V.; Quintana, I.; Cerezo, A.S.; Caramelo, J.J.; Pol-Fachin, L.; Verli, H.; Estevez, J.M.; Ciancia, M. Anticoagulant activity of a unique sulfated pyranosic (1->3)-beta-l-arabinan through direct interaction with thrombin. J. Biol. Chem. 2013, 288, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estevez, J.M.; Fernandez, P.V.; Kasulin, L.; Dupree, P.; Ciancia, M. Chemical and in situ characterization of macromolecular components of the cell walls from the green seaweed Codium fragile. Glycobiology 2009, 19, 212–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa-Rivera, M.G.; Cano-Camacho, H.; Lopez-Romero, E.; Zavala-Paramo, M.G. The Role of Arabinogalactan Type II Degradation in Plant-Microbe Interactions. Front. Microbiol. 2021, 12, 730543. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, L.; Shafee, T.; Johnson, K.L.; Bacic, A.; Classen, B. Arabinogalactan-proteins of Zostera marina L. contain unique glycan structures and provide insight into adaption processes to saline environments. Sci. Rep. 2020, 10, 8232. [Google Scholar] [CrossRef]
- Saeidy, S.; Petera, B.; Pierre, G.; Fenoradosoa, T.A.; Djomdi, D.; Michaud, P.; Delattre, C. Plants arabinogalactans: From structures to physico-chemical and biological properties. Biotechnol. Adv. 2021, 53, 107771. [Google Scholar] [CrossRef]
- Nagel, A.; Conrad, J.; Leitenberger, M.; Carle, R.; Neidhart, S. Structural studies of the arabinogalactans in Mangifera indica L. fruit exudate. Food Hydrocoll. 2016, 61, 555–566. [Google Scholar] [CrossRef]
- Fernández, P.V.; Ciancia, M.; Miravalles, A.B.; Estevez, J.M. Cell-Wall Polymer Mapping in the Coenocytic Macroalga Codium Vermilara (Bryopsidales, Chlorophyta)1. J. Phycol. 2010, 46, 456–465. [Google Scholar] [CrossRef]
- Abou Oualid, H.; Abdellaoui, Y.; Laabd, M.; El Ouardi, M.; Brahmi, Y.; Iazza, M.; Abou Oualid, J. Eco-Efficient Green Seaweed Codium decorticatum Biosorbent for Textile Dyes: Characterization, Mechanism, Recyclability, and RSM Optimization. ACS Omega 2020, 5, 22192–22207. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F.J.N. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Monsigny, M.; Petit, C.; Roche, A.-C. Colorimetric determination of neutral sugars by a resorcinol sulfuric acid micromethod. Anal. Biochem. 1988, 175, 525–530. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G.J.A.b. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Dodgson, K.; Price, R.J.B.J. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [Green Version]
- Pierre, G.; Graber, M.; Rafiliposon, B.A.; Dupuy, C.; Orvain, F.; De Crignis, M.; Maugard, T. Biochemical composition and changes of extracellular polysaccharides (ECPS) produced during microphytobenthic biofilm development (Marennes-Oléron, France). Microb. Ecol. 2012, 63, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Jiang, W.; Bi, Y.; Luo, Y. Postharvest BTH treatment induces resistance of peach (Prunus persica L. cv. Jiubao) fruit to infection by Penicillium expansum and enhances activity of fruit defense mechanisms. Postharvest Biol. Technol. 2005, 35, 263–269. [Google Scholar] [CrossRef]
- Hendrikse, N.M.; Holmberg Larsson, A.; Svensson Gelius, S.; Kuprin, S.; Nordling, E.; Syren, P.O. Exploring the therapeutic potential of modern and ancestral phenylalanine/tyrosine ammonia-lyases as supplementary treatment of hereditary tyrosinemia. Sci. Rep. 2020, 10, 1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- El Modafar, C.; Tantaoui, A.; El Boustani, E. Changes in cell wall-bound phenolic compounds and lignin in roots of date palm cultivars differing in susceptibility to Fusarium oxysporum f. sp albedinis. J. Phytopathol. 2000, 148, 405–411. [Google Scholar] [CrossRef]
- Budini, R.; Tonelli, D.; Girotti, S.J.J.o.A.; Chemistry, F. Analysis of total phenols using the Prussian blue method. J. Agric. Food Chem. 1980, 28, 1236–1238. [Google Scholar] [CrossRef]
- El Modafar, C.; El Boustani, E. Cell wall-bound phenolic acid and lignin contents in date palm as related to its resistance to Fusarium oxysporum. Biol. Plant 2001, 44, 125–130. [Google Scholar] [CrossRef]
- Petera, B.; Delattre, C.; Pierre, G.; Wadouachi, A.; Elboutachfaiti, R.; Engel, E.; Poughon, L.; Michaud, P.; Fenoradosoa, T.A. Characterization of arabinogalactan-rich mucilage from Cereus triangularis cladodes. Carbohydr. Polym. 2015, 127, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Vasi, S.; Vasi, C.; Dugo, G. The role of water in protein’s behavior: The two dynamical crossovers studied by NMR and FTIR techniques. Comput. Struct. Biotechnol. J. 2015, 13, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.L.; Sun, P.N.; Bucheli, P.; Huang, T.H.; Wang, D. FT-IR methodology for quality control of arabinogalactan protein (AGP) extracted from green tea (Camellia sinensis). J. Agric. Food Chem. 2009, 57, 5121–5128. [Google Scholar] [CrossRef]
- Li, N.; Mao, W.; Yan, M.; Liu, X.; Xia, Z.; Wang, S.; Xiao, B.; Chen, C.; Zhang, L.; Cao, S. Structural characterization and anticoagulant activity of a sulfated polysaccharide from the green alga Codium divaricatum. Carbohydr. Polym. 2015, 121, 175–182. [Google Scholar] [CrossRef]
- Aitouguinane, M.; Bouissil, S.; Mouhoub, A.; Rchid, H.; Fendri, I.; Abdelkafi, S.; Ould El-Hadj, M.D.; Boual, Z.; Dubessay, P.; Gardarin, C.; et al. Induction of Natural Defenses in Tomato Seedlings by Using Alginate and Oligoalginates Derivatives Extracted from Moroccan Brown Algae. Mar. Drugs 2020, 18, 521. [Google Scholar] [CrossRef]
- Xin, Z.; Cai, X.; Chen, S.; Luo, Z.; Bian, L.; Li, Z.; Ge, L.; Chen, Z. A Disease Resistance Elicitor Laminarin Enhances Tea Defense against a Piercing Herbivore Empoasca (Matsumurasca) onukii Matsuda. Sci. Rep. 2019, 9, 814. [Google Scholar] [CrossRef]
- Sangha, J.S.; Khan, W.; Ji, X.; Zhang, J.; Mills, A.A.; Critchley, A.T.; Prithiviraj, B. Carrageenans, sulphated polysaccharides of red seaweeds, differentially affect Arabidopsis thaliana resistance to Trichoplusia ni (cabbage looper). PLoS ONE 2011, 6, e26834. [Google Scholar] [CrossRef]
- Agarwal, P.K.; Dangariya, M.; Agarwal, P. Seaweed extracts: Potential biodegradable, environmentally friendly resources for regulating plant defence. Algal Res. 2021, 58, 102363. [Google Scholar] [CrossRef]
- Love, J.; Percival, E. 632. The polysaccharides of the green seaweed Codium fragile. Part II. The water-soluble sulphated polysaccharides. J. Chem. Soc. 1964, 3338–3345. [Google Scholar] [CrossRef]
- Dobrincic, A.; Balbino, S.; Zoric, Z.; Pedisic, S.; Bursac Kovacevic, D.; Elez Garofulic, I.; Dragovic-Uzelac, V. Advanced Technologies for the Extraction of Marine Brown Algal Polysaccharides. Mar. Drugs 2020, 18, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabarsa, M.; Karnjanapratum, S.; Cho, M.; Kim, J.K.; You, S. Molecular characteristics and biological activities of anionic macromolecules from Codium fragile. Int. J. Biol. Macromol. 2013, 59, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Lee, J.-B.; Hayashi, K.; Hayashi, T.J.B.; Bulletin, P. Isolation of sulfated galactan from Codium fragile and its antiviral effect. Pharm. Bull. 2009, 32, 892–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabry, D.A.; Cordeiro, S.L.; Ferreira Silva, C.H.; Cunha Farias, E.H.; Sassaki, G.L.; Nader, H.B.; Oliveira Rocha, H.A. Pharmacological prospection and structural characterization of two purified sulfated and pyruvylated homogalactans from green algae Codium isthmocladum. Carbohydr. Polym. 2019, 222, 115010. [Google Scholar] [CrossRef]
- Přerovská, T.; Henke, S.; Bleha, R.; Spiwok, V.; Gillarová, S.; Yvin, J.C.; Ferrières, V.; Nguema-Ona, E.; Lipovová, P. Arabinogalactan-like Glycoproteins from Ulva lactuca (Chlorophyta) Show Unique Features Compared to Land Plants AGPs. J. Phycol. 2021, 57, 619–635. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, P.V.; Raffo, M.P.; Alberghina, J.; Ciancia, M. Polysaccharides from the green seaweed Codium decorticatum. Structure and cell wall distribution. Carbohydr. Polym. 2015, 117, 836–844. [Google Scholar] [CrossRef]
- Senthilkumar, D.; Jayanthi, S. Partial characterization and anticancer activities of purified glycoprotein extracted from green seaweed Codium decorticatum. J. Funct. Foods 2016, 25, 323–332. [Google Scholar] [CrossRef]
- Walters, D. Plant Defense: Warding Off Attack by Pathogens, Pests and Vertebrate Herbivores; Wiley-Blackwell: Hoboken, NJ, USA, 2011. [Google Scholar]
- Pilate, G.; Dejardin, A.; Leple, J.-C. Field Trials with Lignin–Modified Transgenic Trees. In Lignins—Biosynthesis, Biodegradation and Bioengineering; Academic Press: Cambridge, MA, USA, 2012; pp. 1–36. [Google Scholar]
- Bi, F.; Iqbal, S.E. Dose dependent and time course elicitor activity of Codium elongatum and Ulva lactulus (green algae) of Karachi coast. Pak. J. Bot. 2003, 35, 511–518. [Google Scholar]
- Bi, F.; Iqbal, S. Studies on aqueous extracts of three green algae. J. Pak. J. Bot. 1999, 31, 193–198. [Google Scholar]
- Abouraïcha, E.F.; El Alaoui-Talibi, Z.; Tadlaoui-Ouafi, A.; El Boutachfaiti, R.; Petit, E.; Douira, A.; Courtois, B.; Courtois, J.; El Modafar, C. Glucuronan and oligoglucuronans isolated from green algae activate natural defense responses in apple fruit and reduce postharvest blue and gray mold decay. J. Appl. Phycol. 2016, 29, 471–480. [Google Scholar] [CrossRef]
- Renard-Merlier, D.; Randoux, B.; Nowak, E.; Farcy, F.; Durand, R.; Reignault, P. Iodus 40, salicylic acid, heptanoyl salicylic acid and trehalose exhibit different efficacies and defence targets during a wheat/powdery mildew interaction. Phytochemistry 2007, 68, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Baez, R.V. Lipid Metabolism; BoD-Books on Demand, InTech: Rijeka, Croatia, 2013. [Google Scholar]
- De Wit, P.; Kodde, E. Further characterization and cultivar-specificity of glycoprotein elicitors from culture filtrates and cell walls of Cladosporium fulvum (syn. Fulvia fulva). Physiol. Mol. 1981, 18, 297–314. [Google Scholar] [CrossRef]
- Davis, D.; Low, P.; Heinstein, P.J.P.; Pathology, M.P. Purification of a glycoprotein elicitor of phytoalexin formation fromVerticillium dahliae. Physiol. Mol. 1998, 52, 259–273. [Google Scholar] [CrossRef]
- Pettongkhao, S.; Churngchow, N. Novel Cell Death-Inducing Elicitors from Phytophthora palmivora Promote Infection on Hevea brasiliensis. Phytopathology 2019, 109, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Baillieul, F.; Genetet, I.; Kopp, M.; Saindrenan, P.; Fritig, B.; Kauffmann, S. A new elicitor of the hypersensitive response in tobacco: A fungal glycoprotein elicits cell death, expression of defence genes, production of salicylic acid, and induction of systemic acquired resistance. Plant J. 1995, 8, 551–560. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Qiu, D.; Zeng, H.; Guo, L.; Yang, X. BcGs1, a glycoprotein from Botrytis cinerea, elicits defence response and improves disease resistance in host plants. Biochem. Biophys. Res. Commun. 2015, 457, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qiu, D.; Zeng, H.; Yuan, J.; Mao, J. Purification and characterization of a glycoprotein elicitor from Alternaria tenuissima. World J. Microbiol. Biotechnol. 2009, 25, 2035–2042. [Google Scholar] [CrossRef]
- Han, L.; Sun, Y.; Zhou, X.; Hao, X.; Wu, M.; Zhang, X.; Feng, J. A novel glycoprotein from Streptomyces sp. triggers early responses of plant defense. Pestic. Biochem. Physiol. 2021, 171, 104719. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced systemic resistance (ISR) in plants: Mechanism of action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Menard, R.; Alban, S.; de Ruffray, P.; Jamois, F.; Franz, G.; Fritig, B.; Yvin, J.C.; Kauffmann, S. Beta-1,3 glucan sulfate, but not beta-1,3 glucan, induces the salicylic acid signaling pathway in tobacco and Arabidopsis. Plant Cell 2004, 16, 3020–3032. [Google Scholar] [CrossRef] [Green Version]
- Caillot, S.; Rat, S.; Tavernier, M.-L.; Michaud, P.; Kovensky, J.; Wadouachi, A.; Clément, C.; Baillieul, F.; Petit, E. Native and sulfated oligoglucuronans as elicitors of defence-related responses inducing protection against Botrytis cinerea of Vitis vinifera. Carbohydr. Polym. 2012, 87, 1728–1736. [Google Scholar] [CrossRef]
- Schaffrath, U.; Scheinpflug, H.; Reisener, H.J.P.; Pathology, M.P. An elicitor from Pyricularia oryzae induces resistance responses in rice: Isolation, characterization and physiological properties. Physiol. Mol. 1995, 46, 293–307. [Google Scholar] [CrossRef]
- Sundar, A.R.; Velazhahan, R.; Vidhyasekaran, P. A glycoprotein elicitor isolated from Colletotrichum falcatum induces defense mechanisms in sugarcane leaves and suspension-cultured cells/Ein Glycoprotein aus Colletotrichum falcatum induziert als Elicitor Abwehrmechanismen in Zuckerrohrblättern und Zellkulturen. Plant Dis. Prot. 2002, 109, 601–611. [Google Scholar]
- Parker, J.E.; Schulte, W.; Hahlbrock, K.; Scheel, D. An extracellular glycoprotein from Phytophthora megasperma f. sp. Glycinea elicits phytoalexin synthesis in cultured parsley cells and protoplasts. Mol. Plant Microbe Interact 1991, 4, 19–27. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Corio-Costet, M.-F.; Stadnik, M.J.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.-T.; Dumas, B. An Ulva armoricana extract protects plants against three powdery mildew pathogens. Eur. J. Plant Pathol. 2011, 131, 393–401. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-Based Biostimulants: Sustainable Applications in Agriculture for the Stimulation of Plant Growth, Stress Tolerance, and Disease Management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [Green Version]










| AGB 2 | AGP 3 | |
|---|---|---|
| Yields (% w/w) | 11 | 5 |
| Total sugars (% w/w) | 37.67 | 48.38 |
| Neutral sugars (% w/w) | 37.56 | 45.68 |
| Uronic acids (% w/w) | 3.05 | 4.04 |
| Sulfate groups (% w/w) | 22.68 | 13.21 |
| Proteins (% w/w) | 20.03 | 11.89 |
| Phenolic compounds (% w/w) | Trace 1 | Trace 1 |
| AGB 4 | AGP 5 | |
|---|---|---|
| Mn 1 (g·mol−1) | 711 × 103 (±1.4%) | 1295 × 103 (±0.9%) |
| Mw 2 (g·mol−1) | 2173 × 103 (±0.4%) | 2042 × 103 (±0.7%) |
| Đ 3 | 3.1 (±1.4%) | 1.6 (±1.2%) |
| Intrinsic viscosity (mL·g−1) | 266.7 (±3.0%) | 560.6 (±4.0%) |
| Monosaccharides (% Molar Ratio) | AGB 1 | AGP 2 |
|---|---|---|
| Galactose (Gal) | 46.07 ± 1.46 | 51.55 ± 2.67 |
| Glucose (Glc) | 38.78 ± 0.24 | 24.56 ± 1.05 |
| Arabinose (Ara) | 12.08 ± 1 | 17.32 ± 1.33 |
| Xylose (Xyl) | 1.70 ± 0.23 | 3.93 ± 0.61 |
| Rhamnose (Rha) | 1.37 ± 0.13 | 2.63 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitouguinane, M.; El Alaoui-Talibi, Z.; Rchid, H.; Fendri, I.; Abdelkafi, S.; Ould El-Hadj, M.D.; Boual, Z.; Dubessay, P.; Michaud, P.; Le Cerf, D.; et al. A Novel Sulfated Glycoprotein Elicitor Extracted from the Moroccan Green Seaweed Codium decorticatum Induces Natural Defenses in Tomato. Appl. Sci. 2022, 12, 3643. https://doi.org/10.3390/app12073643
Aitouguinane M, El Alaoui-Talibi Z, Rchid H, Fendri I, Abdelkafi S, Ould El-Hadj MD, Boual Z, Dubessay P, Michaud P, Le Cerf D, et al. A Novel Sulfated Glycoprotein Elicitor Extracted from the Moroccan Green Seaweed Codium decorticatum Induces Natural Defenses in Tomato. Applied Sciences. 2022; 12(7):3643. https://doi.org/10.3390/app12073643
Chicago/Turabian StyleAitouguinane, Meriem, Zainab El Alaoui-Talibi, Halima Rchid, Imen Fendri, Slim Abdelkafi, Mohamed Didi Ould El-Hadj, Zakaria Boual, Pascal Dubessay, Philippe Michaud, Didier Le Cerf, and et al. 2022. "A Novel Sulfated Glycoprotein Elicitor Extracted from the Moroccan Green Seaweed Codium decorticatum Induces Natural Defenses in Tomato" Applied Sciences 12, no. 7: 3643. https://doi.org/10.3390/app12073643
APA StyleAitouguinane, M., El Alaoui-Talibi, Z., Rchid, H., Fendri, I., Abdelkafi, S., Ould El-Hadj, M. D., Boual, Z., Dubessay, P., Michaud, P., Le Cerf, D., Rihouey, C., Pierre, G., Delattre, C., & El Modafar, C. (2022). A Novel Sulfated Glycoprotein Elicitor Extracted from the Moroccan Green Seaweed Codium decorticatum Induces Natural Defenses in Tomato. Applied Sciences, 12(7), 3643. https://doi.org/10.3390/app12073643