Evolving Strategies and Materials for Scaffold Development in Regenerative Dentistry
Abstract
:1. Introduction
1.1. Historical Background and Significance of Scaffold Development
1.2. Timeline of Significant Advancements in Scaffold Development in Dentistry
- Early 1990s
- Late 1990s–Early 2000s
- Mid-2000s
- Late 2000s–Early 2010s
- Mid-2010s–Present
1.3. Aim and Scope of This Review
2. Materials and Methods
2.1. Evaluation of the Most Impactful Papers Based on Scaffold Manufacturing
2.2. Evaluation of the Most Commonly Used Materials
“What materials were used in scaffold manufacturing in regenerative dentistry during last ten years? Provide a timeline with highlighting the most popular/researched material of each particular year.”
3. Results
4. Discussion
4.1. Mesenchymal Stem Cells and Their Application in Regenerative Dentistry
4.2. Materials for Scaffold Fabrication in Regenerative Dentistry
4.3. 3D Bioprinting in Regenerative Dentistry
4.4. Novel Techniques and Modifications in Scaffold Fabrication
4.5. Whole Tooth Regeneration
4.6. Future of Scaffold Approaches
- Decellularized matrices are natural scaffolds created by removing cells from tissue. They are biocompatible, biodegradable, and can be tailored for specific tissue regeneration, such as using heart matrices to regenerate heart muscle [141].
- Hydrogels are soft, flexible materials made from natural (e.g., collagen) or synthetic polymers. Their customizable properties like stiffness, degradation, and cell adhesion make them versatile for supporting a wide range of tissue growth.
- 3D printing is a technology that can be used to create complex scaffolds with intricate structures that mimic the natural extracellular matrix (ECM) of tissues. This can help to improve the ability of scaffolds to support cell growth and differentiation. 3D printing is also a relatively rapid and efficient process, which can make it a more cost-effective way to produce scaffolds or even personalized medical appliances [151,169].
- Adding growth factors and other molecules to scaffolds improves their performance in tissue regeneration by promoting cell growth, differentiation, and tissue formation.
- Self-assembling scaffolds are materials that can spontaneously assemble into complex structures without the need for external forces. This can lead to the formation of scaffolds that are highly porous and interconnected, which is ideal for supporting cell growth and tissue regeneration.
- Bioactive materials are materials that can release bioactive molecules, such as growth factors and signaling molecules, over time. This can help to promote cell growth, differentiation, and tissue formation. Bioactive materials can also be used to deliver drugs and other therapeutic agents to cells and tissues.
- Microfabrication is a technology that can be used to create scaffolds with micrometer-scale features. This can be used to control the size and shape of pores in scaffolds, which can affect the ability of cells to adhere and grow on the scaffold.
- Electromagnetic patterning is a technology that can be used to create scaffolds with patterns of electrical charges. This can be used to attract and guide cells to specific locations on the scaffold.
- Bioprinting is a technology that can be used to create scaffolds with complex structures using living cells. This can be used to create scaffolds that are more similar to natural tissues and that can support the growth of a wider variety of cell types.
5. Conclusions
5.1. Overall Conclusions
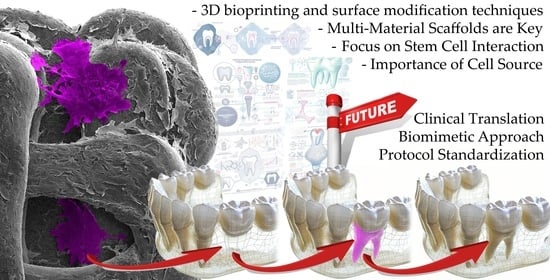
- Multi-Material Scaffolds are Key: The most impactful research emphasizes the need for combining various materials in biocompatible scaffolds to achieve tailored properties and optimal biological responses in hard tissue regeneration.
- Focus on Stem Cell Interaction: Studies with the highest impact explore how scaffold materials influence stem cell proliferation, differentiation, and behavior. Understanding these material-cell interactions is crucial for developing successful therapies.
- Novel Modifications are Promising: Advancements in nanotechnology, 3D bioprinting, and surface modification techniques have the potential to revolutionize scaffold design, increasing their efficiency and customization for regenerative dentistry.
5.2. Specific Conclusions
- Graphene, Chitosan, and Composites: Graphene demonstrates antibacterial properties and cellular stimulation, making it a valuable candidate. Chitosan, while needing improvement on its own, shows promise when combined with other materials like hydroxyapatite.
- Bioprinting for Tailored Solutions: 3D bioprinting shows tremendous promise for creating patient-specific scaffolds, driving greater customization and success rates in dental tissue regeneration. This includes bioprinting of cells, matrix materials, and entire tooth structures.
- Importance of Cell Source: Exploration of mesenchymal stem cells (MSCs) from different sources (dental pulp, bone marrow, adipose tissue) in conjunction with scaffolds is highly significant for determining optimal cell-material pairings for specific applications.
- Newer Materials Emerge: Bioactive glasses, boron-doped biomaterials, and unique composites hold promise for enhanced bone and tooth regeneration.
5.3. Future Directions
- Biomimetic Approaches: Further emphasis on biomimetic principles, mimicking natural tissue structures and compositions, will likely drive future scaffold material and design innovations.
- Clinical Translation: A strong need exists to translate promising laboratory findings on scaffold-based materials and approaches into clinical dentistry, paving the way for more effective and available treatments.
- In-depth Material Investigations: Continued in-depth research on biocompatibility, degradation rates, cell interactions, and potential cytotoxicity of novel and complex scaffold materials is essential.
- Standardization: As the field matures, standardization of protocols, evaluation metrics, and reporting methods becomes critical for comparing research findings and accelerating clinical adoption.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | artificial intelligence |
| 3D | three dimensional |
| PLA | polylactic acid |
| PGA | polyglycolic acid |
| HAp | hydroxyapatite |
| HA | hyaluronic acid |
| TCP | tri calcium phosphate |
| LLM | large language model |
| PLGA | polylactic-co-glycolic acid |
| PCL | polycaprolactone |
| CPC | calcium phosphate cements |
| BCP | biphasic calcium phosphate |
| MSCs | mesenchymal stem cells |
| ECM | extracellular matrix |
| DSCs | dental stem cells |
| BMP-2 | bone morphogenic protein 2 |
| BMP-7 | bone morphogenic protein 7 |
| MMP-8 | matrix metalloproteinase 8 |
| b-FGF | basic-fibroblast growth factor |
| BMSCs | bone marrow stem cells |
| ADSCs | adipose tissue stem cells |
| iPSCs | induced pluripotent stem cells |
| SHEDs | human exfoliated deciduous teeth stem cells |
| APSCs | apical papilla stem cells |
| ALP | alkaline phosphatase |
| hPCy-MSCs | human periapical cyst derived mesenchymal stem cells |
| DCPD | dicalcium phosphate dihydrate |
| CaSi | calcium silicate |
| DMP-1 | dentin matrix protein-1 |
| RUNX-2 | runt-related transcription factor 2 |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide |
| DPSCs | dental pulp stem cells |
| Gel-MA | gelatine methacrylate |
| SV | simvastin |
| DBBM | deproteinized bovine bone mineral |
| Micro-CT | micro computer tomography |
| USAG-1 | uterine sensitization-associated gene-1 |
| 4D | four dimensional |
| PEG | polyethylene glycol |
References
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Meyer, U. The History of Tissue Engineering and Regenerative Medicine in Perspective. In Fundamentals of Tissue Engineering and Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2009; pp. 5–12. [Google Scholar]
- Ricci, J.L.; Terracio, L. Where Is Dentistry in Regenerative Medicine? Int. Dent. J. 2011, 61, 2–10. [Google Scholar] [CrossRef]
- Kaiser, L.R. The Future of Multihospital Systems. Top. Health Care Financ. 1992, 18, 32–45. [Google Scholar]
- Vacanti, C.A. The History of Tissue Engineering. J. Cell Mol. Med. 2006, 10, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Aghali, A. Craniofacial Bone Tissue Engineering: Current Approaches and Potential Therapy. Cells 2021, 10, 2993. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, M.; Fujioka-Kobayashi, M.; Yoneyama, Y.; Inada, R.; Satomi, T. Regenerative Potential of Solid Bone Marrow Aspirate Concentrate Compared with Platelet-Rich Fibrin. Tissue Eng. Part A 2022, 28, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Thalakiriyawa, D.S.; Dissanayaka, W.L. Advances in Regenerative Dentistry Approaches: An Update. Int. Dent. J. 2023, 74, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ramezanzade, S.; Aeinehvand, M.; Ziaei, H.; Khurshid, Z.; Keyhan, S.O.; Fallahi, H.R.; Melville, J.C.; Saeinasab, M.; Sefat, F. Reconstruction of Critical Sized Maxillofacial Defects Using Composite Allogeneic Tissue Engineering: Systematic Review of Current Literature. Biomimetics 2023, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Quirino, L.C.; de Azambuja Carvalho, P.H.; Neto, R.T.A.; Comachio, C.A.; Monteiro, N.G.; Ervolino-Silva, A.C.; Okamoto, R.; Pereira-Filho, V.A. Polydioxanone Membrane Compared with Collagen Membrane for Bone Regeneration. Polymers 2023, 15, 868. [Google Scholar] [CrossRef]
- Verykokou, S.; Ioannidis, C.; Angelopoulos, C. CBCT-Based Design of Patient-Specific 3D Bone Grafts for Periodontal Regeneration. J. Clin. Med. 2023, 12, 5023. [Google Scholar] [CrossRef]
- Malcangi, G.; Patano, A.; Guglielmo, M.; Sardano, R.; Palmieri, G.; Di Pede, C.; de Ruvo, E.; Inchingolo, A.M.A.D.; Mancini, A.; Inchingolo, F.; et al. Precision Medicine in Oral Health and Diseases: A Systematic Review. J. Pers. Med. 2023, 13, 725. [Google Scholar] [CrossRef]
- Granz, C.L.; Gorji, A. Dental Stem Cells: The Role of Biomaterials and Scaffolds in Developing Novel Therapeutic Strategies. World J. Stem Cells 2020, 12, 897–921. [Google Scholar] [CrossRef]
- Tollemar, V.; Collier, Z.J.; Mohammed, M.K.; Lee, M.J.; Ameer, G.A.; Reid, R.R. Stem Cells, Growth Factors and Scaffolds in Craniofacial Regenerative Medicine. Genes. Dis. 2016, 3, 56–71. [Google Scholar] [CrossRef]
- Suh, H. Tissue Restoration, Tissue Engineering and Regenerative Medicine. Yonsei Med. J. 2000, 41, 681–684. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Agrawal, C.M.; Barber, F.A.; Burkhart, S.S. Orthopaedic Applications for PLA-PGA Biodegradable Polymers. Arthrosc. J. Arthrosc. Relat. Surg. 1998, 14, 726–737. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, Toxicity, Biocompatibility and Clinical Applications of Polylactic Acid/ Polyglycolic Acid Copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Sugiaman, V.K.; Jeffrey; Naliani, S.; Pranata, N.; Djuanda, R.; Saputri, R.I. Polymeric Scaffolds Used in Dental Pulp Regeneration by Tissue Engineering Approach. Polymers 2023, 15, 1082. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, B.; Figat, R.; Kolmas, J. Biomimetic Apatite/Natural Polymer Composite Granules as Multifunctional Dental Tissue Regenerative Material. Int. J. Mol. Sci. 2023, 24, 16751. [Google Scholar] [CrossRef] [PubMed]
- Mohd, N.; Razali, M.; Fauzi, M.B.; Abu Kasim, N.H. In Vitro and In Vivo Biological Assessments of 3D-Bioprinted Scaffolds for Dental Applications. Int. J. Mol. Sci. 2023, 24, 12881. [Google Scholar] [CrossRef] [PubMed]
- Shopova, D.; Mihaylova, A.; Yaneva, A.; Bakova, D. Advancing Dentistry through Bioprinting: Personalization of Oral Tissues. J. Funct. Biomater. 2023, 14, 530. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Kuznetsova, A.V.; Popova, O.P.; Danilova, T.I.; Latyshev, A.V.; Yanushevich, O.O. Influence of Extracellular Matrix Components on the Differentiation of Periodontal Ligament Stem Cells in Collagen I Hydrogel. Cells 2023, 12, 2335. [Google Scholar] [CrossRef] [PubMed]
- Eshwar, S.; Konuganti, K.; Manvi, S.; Bharadwaj, A.N.; Sajjan, S.; Boregowda, S.S.; Jain, V. Evaluation of Osteogenic Potential of Fucoidan Containing Chitosan Hydrogel in the Treatment of Periodontal Intra-Bony Defects—A Randomized Clinical Trial. Gels 2023, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Atia, G.A.N.; Shalaby, H.K.; Ali, N.G.; Morsy, S.M.; Ghobashy, M.M.; Attia, H.A.N.; Barai, P.; Nady, N.; Kodous, A.S.; Barai, H.R. New Challenges and Prospective Applications of Three-Dimensional Bioactive Polymeric Hydrogels in Oral and Craniofacial Tissue Engineering: A Narrative Review. Pharmaceuticals 2023, 16, 702. [Google Scholar] [CrossRef] [PubMed]
- Dal-Fabbro, R.; Huang, Y.-C.; Toledo, P.T.A.; Capalbo, L.C.; Coleman, R.M.; Sasaki, H.; Fenno, J.C.; Bottino, M.C. Injectable Methacrylated Gelatin Hydrogel for Safe Sodium Hypochlorite Delivery in Endodontics. Gels 2023, 9, 897. [Google Scholar] [CrossRef]
- Safari, B.; Davaran, S.; Aghanejad, A. Osteogenic Potential of the Growth Factors and Bioactive Molecules in Bone Regeneration. Int. J. Biol. Macromol. 2021, 175, 544–557. [Google Scholar] [CrossRef]
- Heng, B.C.; Bai, Y.; Li, X.; Meng, Y.; Zhang, X.; Deng, X. Signaling Pathways Implicated in Enhanced Stem/Progenitor Cell Differentiation on Electroactive Scaffolds. Smart Mater. Med. 2022, 3, 4–11. [Google Scholar] [CrossRef]
- Li, R.; Liu, K.; Huang, X.; Li, D.; Ding, J.; Liu, B.; Chen, X.; Li, R.; Liu, K.; Liu, B.; et al. Bioactive Materials Promote Wound Healing through Modulation of Cell Behaviors. Adv. Sci. 2022, 9, 2105152. [Google Scholar] [CrossRef]
- Safari, B.; Aghanejad, A.; Roshangar, L.; Davaran, S. Osteogenic Effects of the Bioactive Small Molecules and Minerals in the Scaffold-Based Bone Tissue Engineering. Colloids Surf. B Biointerfaces 2021, 198, 111462. [Google Scholar] [CrossRef]
- Strunga, M.; Urban, R.; Surovková, J.; Thurzo, A. Artificial Intelligence Systems Assisting in the Assessment of the Course and Retention of Orthodontic Treatment. Healthcare 2023, 11, 683. [Google Scholar] [CrossRef] [PubMed]
- Thurzo, A.; Stanko, P.; Urbanova, W.; Lysy, J.; Suchancova, B.; Makovnik, M.; Javorka, V. The WEB 2.0 Induced Paradigm Shift in the e-Learning and the Role of Crowdsourcing in Dental Education. Bratisl. Lek. Listy 2010, 111, 168–175. [Google Scholar] [PubMed]
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Tavakkoli Yaraki, M.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and Its Derivatives: Opportunities and Challenges in Dentistry. Mater. Sci. Eng. C 2019, 102, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Spagnuolo, G.; Codispoti, B.; Zamparini, F.; Zhang, A.; Esposti, M.D.; Aparicio, C.; Rengo, C.; Nuzzolese, M.; Manzoli, L.; et al. PLA-Based Mineral-Doped Scaffolds Seeded with Human Periapical Cyst-Derived MSCs: A Promising Tool for Regenerative Healing in Dentistry. Materials 2019, 12, 597. [Google Scholar] [CrossRef] [PubMed]
- Ducret, M.; Montembault, A.; Josse, J.; Pasdeloup, M.; Celle, A.; Benchrih, R.; Mallein-Gerin, F.; Alliot-Licht, B.; David, L.; Farges, J.C. Design and Characterization of a Chitosan-Enriched Fibrin Hydrogel for Human Dental Pulp Regeneration. Dent. Mater. 2019, 35, 523–533. [Google Scholar] [CrossRef]
- Matichescu, A.; Ardelean, L.C.; Rusu, L.C.; Craciun, D.; Bratu, E.A.; Babucea, M.; Leretter, M. Advanced Biomaterials and Techniques for Oral Tissue Engineering and Regeneration—A Review. Materials 2020, 13, 5303. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, L.; Yang, B.; Tian, W. Three-Dimensional Printing Biotechnology for the Regeneration of the Tooth and Tooth-Supporting Tissues. Biotechnol. Bioeng. 2019, 116, 452–468. [Google Scholar] [CrossRef]
- Yelick, P.C.; Sharpe, P.T. Tooth Bioengineering and Regenerative Dentistry. J. Dent. Res. 2019, 98, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Prahasanti, C.; Nugraha, A.P.; Saskianti, T.; Suardita, K.; Riawan, W.; Ernawati, D.S. Exfoliated Human Deciduous Tooth Stem Cells Incorporating Carbonate Apatite Scaffold Enhance BMP-2, BMP-7 and Attenuate MMP-8 Expression During Initial Alveolar Bone Remodeling in Wistar Rats (Rattus norvegicus). Clin. Cosmet. Investig. Dent. 2020, 12, 79–85. [Google Scholar] [CrossRef]
- Alipour, M.; Firouzi, N.; Aghazadeh, Z.; Samiei, M.; Montazersaheb, S.; Khoshfetrat, A.B.; Aghazadeh, M. The Osteogenic Differentiation of Human Dental Pulp Stem Cells in Alginate-Gelatin/Nano-Hydroxyapatite Microcapsules. BMC Biotechnol. 2021, 21, 6. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef]
- Baranova, J.; Büchner, D.; Götz, W.; Schulze, M.; Tobiasch, E. Tooth Formation: Are the Hardest Tissues of Human Body Hard to Regenerate? Int. J. Mol. Sci. 2020, 21, 4031. [Google Scholar] [CrossRef]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of Collagen Nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef]
- Samiei, M.; Alipour, M.; Khezri, K.; Saadat, Y.R.; Forouhandeh, H.; Abdolahinia, E.D.; Vahed, S.Z.; Sharifi, S.; Dizaj, S.M. Application of Collagen and Mesenchymal Stem Cells in Regenerative Dentistry. Curr. Stem Cell Res. Ther. 2021, 17, 606–620. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The Development of Collagen Based Composite Scaffolds for Bone Regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef]
- Monteiro, N.; Thrivikraman, G.; Athirasala, A.; Tahayeri, A.; França, C.M.; Ferracane, J.L.; Bertassoni, L.E. Photopolymerization of Cell-Laden Gelatin Methacryloyl Hydrogels Using a Dental Curing Light for Regenerative Dentistry. Dent. Mater. 2018, 34, 389–399. [Google Scholar] [CrossRef]
- Parthiban, S.P.; He, W.; Monteiro, N.; Athirasala, A.; França, C.M.; Bertassoni, L.E. Engineering Pericyte-Supported Microvascular Capillaries in Cell-Laden Hydrogels Using Stem Cells from the Bone Marrow, Dental Pulp and Dental Apical Papilla. Sci. Rep. 2020, 10, 21579. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Liu, Y.; Zhang, C.J.; Zhang, C.; Zhu, P. Alginate/Gelatin Blended Hydrogel Fibers Cross-Linked by Ca2+ and Oxidized Starch: Preparation and Properties. Mater. Sci. Eng. C 2019, 99, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Ren, Q.; Li, Z.; Zhang, L. Chitosan-Based Biomimetically Mineralized Composite Materials in Human Hard Tissue Repair. Molecules 2020, 25, 4785. [Google Scholar] [CrossRef]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan Biomaterials for Current and Potential Dental Applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef]
- Zhang, Y.; Venugopal, J.R.; El-Turki, A.; Ramakrishna, S.; Su, B.; Lim, C.T. Electrospun Biomimetic Nanocomposite Nanofibers of Hydroxyapatite/Chitosan for Bone Tissue Engineering. Biomaterials 2008, 29, 4314–4322. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Liu, Y.; Liu, X.; Wang, Z.; Wan, J.; Yu, X.; Wang, S. Multifunctional Hydrogel/Platelet-Rich Fibrin/Nanofibers Scaffolds with Cell Barrier and Osteogenesis for Guided Tissue Regeneration/Guided Bone Regeneration Applications. Int. J. Biol. Macromol. 2023, 253, 126960. [Google Scholar] [CrossRef]
- Mylonaki, I.; Allémann, É.; Saucy, F.; Haefliger, J.A.; Delie, F.; Jordan, O. Perivascular Medical Devices and Drug Delivery Systems: Making the Right Choices. Biomaterials 2017, 128, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M. Biomaterials for Bone Tissue Engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Cordonnier, T.; Sohier, J.; Rosset, P.; Layrolle, P. Biomimetic Materials for Bone Tissue Engineering—State of the Art and Future Trends. Adv. Eng. Mater. 2011, 13, B135–B150. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(Lactic-Co-Glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.V.; Vaz, C.M.; Tomlins, P.E.; Mikhalovska, L.; Mikhalovsky, S.; James, S.; Vadgama, P. Physical Characterisation of a Polycaprolactone Tissue Scaffold. In Surface Chemistry in Biomedical and Environmental Science; Springer: Dordrecht, The Netherlands, 2006; pp. 215–228. [Google Scholar] [CrossRef]
- Vance, R.J.; Miller, D.C.; Thapa, A.; Haberstroh, K.M.; Webster, T.J. Decreased Fibroblast Cell Density on Chemically Degraded Poly-Lactic-Co-Glycolic Acid, Polyurethane, and Polycaprolactone. Biomaterials 2004, 25, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Taylor, C. Chemical, Physical and Biological Properties of Alginates and Their Biomedical Implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and Alginate Composites for Biomedical Applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Gwon, K.; Kim, E.; Tae, G. Heparin-Hyaluronic Acid Hydrogel in Support of Cellular Activities of 3D Encapsulated Adipose Derived Stem Cells. Acta Biomater. 2017, 49, 284–295. [Google Scholar] [CrossRef]
- Almeida, L.D.F.; Babo, P.S.; Silva, C.R.; Rodrigues, M.T.; Hebling, J.; Reis, R.L.; Gomes, M.E. Hyaluronic Acid Hydrogels Incorporating Platelet Lysate Enhance Human Pulp Cell Proliferation and Differentiation. J. Mater. Sci. Mater. Med. 2018, 29, 88. [Google Scholar] [CrossRef]
- Burdick, J.A.; Chung, C. Influence of Three-Dimensional Hyaluronic Acid Microenvironments on Mesenchymal Stem Cell Chondrogenesis. Tissue Eng. Part A 2009, 15, 243–254. [Google Scholar] [CrossRef]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef]
- Wu, D.T.; Munguia-Lopez, J.G.; Cho, Y.W.; Ma, X.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric Scaffolds for Dental, Oral, and Craniofacial Regenerative Medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef]
- Dahiya, P.; Kamal, R. Hyaluronic Acid: A Boon in Periodontal Therapy. N. Am. J. Med. Sci. 2013, 5, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, E.; Eftekhari, A.; Dizaj, S.M.; Sharifi, S.; Mokhtarpour, M.; Nasibova, A.N.; Khalilov, R.; Samiei, M. The Effect of Hyaluronic Acid Hydrogels on Dental Pulp Stem Cells Behavior. Int. J. Biol. Macromol. 2019, 140, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, N.; Mohammed, H.; Al-Hadeethi, Y.; Bakry, A.S.; Umar, A.; Hussein, M.A.; Abbassy, M.A.; Vaidya, K.G.; Al Berakdar, G.; Mkawi, E.M.; et al. Silica-Based Bioactive Glasses and Their Applications in Hard Tissue Regeneration: A Review. Pharmaceuticals 2021, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Mosaddada, S.A.; Yazdaniana, M.; Tebyanian, H.; Tahmasebia, E.; Yazdanianb, A.; Seifalianc, A.; Tavakolizadehd, M. Fabrication and Properties of Developed Collagen/Strontium-Doped Bioglass Scaffolds for Bone Tissue Engineering. J. Mater. Res. Technol. 2020, 9, 14799–14817. [Google Scholar] [CrossRef]
- Paschos, N.K.; Brown, W.E.; Eswaramoorthy, R.; Hu, J.C.; Athanasiou, K.A. Advances in Tissue Engineering through Stem Cell-Based Co-Culture. J. Tissue Eng. Regen. Med. 2015, 9, 488–503. [Google Scholar] [CrossRef]
- Moonesi Rad, R.; Pazarçeviren, E.; Ece Akgün, E.; Evis, Z.; Keskin, D.; Şahin, S.; Tezcaner, A. In Vitro Performance of a Nanobiocomposite Scaffold Containing Boron-Modified Bioactive Glass Nanoparticles for Dentin Regeneration. J. Biomater. Appl. 2019, 33, 834–853. [Google Scholar] [CrossRef] [PubMed]
- Lowe, B.; Ottensmeyer, M.P.; Xu, C.; He, Y.; Ye, Q.; Troulis, M.J. The Regenerative Applicability of Bioactive Glass and Beta-Tricalcium Phosphate in Bone Tissue Engineering: A Transformation Perspective. J. Funct. Biomater. 2019, 10, 16. [Google Scholar] [CrossRef]
- Vaiani, L.; Boccaccio, A.; Uva, A.E.; Palumbo, G.; Piccininni, A.; Guglielmi, P.; Cantore, S.; Santacroce, L.; Charitos, I.A.; Ballini, A. Ceramic Materials for Biomedical Applications: An Overview on Properties and Fabrication Processes. J. Funct. Biomater. 2023, 14, 146. [Google Scholar] [CrossRef]
- Kim, H.Y.; Cooley, V.; Kim, E.J.; Li, S.; Lee, J.M.; Sheyfer, D.; Liu, W.; Klein, O.D.; Joester, D.; Jung, H.S. Adult Dental Epithelial Stem Cell-Derived Organoids Deposit Hydroxylapatite Biomineral. Int. J. Oral. Sci. 2023, 15, 55. [Google Scholar] [CrossRef]
- Zawadzka-Knefel, A.; Rusak, A.; Mrozowska, M.; Machałowski, T.; Żak, A.; Haczkiewicz-Leśniak, K.; Kulus, M.; Kuropka, P.; Podhorska-Okołów, M.; Skośkiewicz-Malinowska, K. Chitin Scaffolds Derived from the Marine Demosponge Aplysina Fistularis Stimulate the Differentiation of Dental Pulp Stem Cells. Front. Bioeng. Biotechnol. 2023, 11, 1254506. [Google Scholar] [CrossRef]
- Gupta, P.; Sharma, S.; Jabin, S.; Jadoun, S. Chitosan Nanocomposite for Tissue Engineering and Regenerative Medicine: A Review. Int. J. Biol. Macromol. 2023, 254, 127660. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Park, S.; Choi, J.; Vu, T.T.H.; Doan, V.H.M.; Vo, T.T.; Lee, B.; Oh, J. Hydroxyapatite: A Journey from Biomaterials to Advanced Functional Materials. Adv. Colloid. Interface Sci. 2023, 321, 103013. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, Y.; Ji, L.; Geng, Z.; Wang, J.; Liu, C. Calcium Phosphate-Based Materials Regulate Osteoclast-Mediated Osseointegration. Bioact. Mater. 2021, 6, 4517–4530. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Yan, D.; Zhou, Q.; Wu, Z.; Weng, S.; Boodhun, V.; Bai, B.; Shen, Z.; Tang, J.; Chen, L.; et al. The Fast Degradation of β-TCP Ceramics Facilitates Healing of Bone Defects by the Combination of BMP-2 and Teriparatide. Biomed. Pharmacother. 2019, 112, 108578. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-Tricalcium Phosphate for Bone Substitution: Synthesis and Properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Segura-Egea, J.J.; Díaz-Cuenca, A. Sol–Gel Technologies to Obtain Advanced Bioceramics for Dental Therapeutics. Molecules 2023, 28, 6967. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Xu, X. Bioceramics in Endodontics: Updates and Future Perspectives. Bioengineering 2023, 10, 354. [Google Scholar] [CrossRef] [PubMed]
- Fariña, N.M.; Guzón, F.M.; Peña, M.L.; Cantalapiedra, A.G. In Vivo Behaviour of Two Different Biphasic Ceramic Implanted in Mandibular Bone of Dogs. J. Mater. Sci. Mater. Med. 2008, 19, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wan, L.; Xiao, Y.; Wang, Y.; Wu, Z.; Guo, W.; Yang, H.; Hu, T. Enhanced Reparative Dentinogenesis of Biphasic Calcium Phosphate Ceramics Containing Calcium-Deficient Hydroxyapatite (CDHA) and Strontium-Incorporated CDHA in Direct Pulp Capping. Mater. Today Commun. 2022, 33, 104231. [Google Scholar] [CrossRef]
- Daculsi, G.; Legeros, R.Z.; Nery, E.; Lynch, K.; Kerebel, B. Transformation of Biphasic Calcium Phosphate Ceramics in Vivo: Ultrastructural and Physicochemical Characterization. J. Biomed. Mater. Res. 1989, 23, 883–894. [Google Scholar] [CrossRef]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic Calcium Phosphate Ceramics for Bone Reconstruction: A Review of Biological Response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Sees, T.M.; Van Den Doel, M.A.; Van Blitterswijk, C.A.; De Groot, K. Osteoinduction by Biomaterials—Physicochemical and Structural Influences. J. Biomed. Mater. Res. A 2006, 77A, 747–762. [Google Scholar] [CrossRef]
- Gauthier, O.; Bouler, J.M.; Aguado, E.; Pilet, P.; Daculsi, G. Macroporous Biphasic Calcium Phosphate Ceramics: Influence of Macropore Diameter and Macroporosity Percentage on Bone Ingrowth. Biomaterials 1998, 19, 133–139. [Google Scholar] [CrossRef]
- Fathi, M.; Kholtei, A.; EL Youbi, S.; Chafik El Idrissi, B. Setting Properties of Calcium Phosphate Bone Cement. Mater. Today Proc. 2019, 13, 876–881. [Google Scholar] [CrossRef]
- Fukase, Y.; Eanes, E.D.; Takagp, S.; Chow, L.C.; Brown, W.E. Setting Reactions and Compressive Strengths of Calcium Phosphate Cements. J. Dent. Res. 1990, 69, 1852–1856. [Google Scholar] [CrossRef]
- Julien, M.; Khairoun, I.; LeGeros, R.Z.; Delplace, S.; Pilet, P.; Weiss, P.; Daculsi, G.; Bouler, J.M.; Guicheux, J. Physico-Chemical–Mechanical and in Vitro Biological Properties of Calcium Phosphate Cements with Doped Amorphous Calcium Phosphates. Biomaterials 2007, 28, 956–965. [Google Scholar] [CrossRef]
- Cama, G. Calcium Phosphate Cements for Bone Regeneration. In Biomaterials for Bone Regeneration: Novel Techniques and Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–25. [Google Scholar] [CrossRef]
- Ambard, A.J.; Mueninghoff, L. Calcium Phosphate Cement: Review of Mechanical and Biological Properties. J. Prosthodont. 2006, 15, 321–328. [Google Scholar] [CrossRef]
- Mishchenko, O.; Yanovska, A.; Kosinov, O.; Maksymov, D.; Moskalenko, R.; Ramanavicius, A.; Pogorielov, M. Synthetic Calcium–Phosphate Materials for Bone Grafting. Polymers 2023, 15, 3822. [Google Scholar] [CrossRef] [PubMed]
- Roi, A.; Roi, C.; Negruțiu, M.L.; Rusu, L.C.; Riviș, M. Mesenchymal Stem Cells Derived from Human Periapical Cysts and Their Implications in Regenerative Medicine. Biomedicines 2023, 11, 2436. [Google Scholar] [CrossRef]
- Cabaña-Muñoz, M.E.; Pelaz Fernández, M.J.; Parmigiani-Cabaña, J.M.; Parmigiani-Izquierdo, J.M.; Merino, J.J. Adult Mesenchymal Stem Cells from Oral Cavity and Surrounding Areas: Types and Biomedical Applications. Pharmaceutics 2023, 15, 2109. [Google Scholar] [CrossRef] [PubMed]
- Angelis, N.D.; Amaroli, A.; Lagazzo, A.; Barberis, F.; Zarro, P.R.; Cappelli, A.; Sabbieti, M.G.; Agas, D. Multipotent Mesenchymal Cells Homing and Differentiation on Poly(ε-Caprolactone) Blended with 20% Tricalcium Phosphate and Polylactic Acid Incorporating 10% Hydroxyapatite 3D-Printed Scaffolds via a Commercial Fused Deposition Modeling 3D Device. Biology 2023, 12, 1474. [Google Scholar] [CrossRef]
- Keyhan, S.O.; Fallahi, H.; Jahangirnia, A.; Mohammad, S.; Masoumi, R.; Khosravi, M.H.; Hosein Amirzade-Iranaq, M. Tissue Engineering Applications in Maxillofacial Surgery; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Oshima, M.; Tsuji, T.; Oshima, M.; Tsuji, T. Whole Tooth Regeneration Using a Bioengineered Tooth. In New Trends in Tissue Engineering and Regenerative Medicine—Official Book of the Japanese Society for Regenerative Medicine; IntechOpen: London, UK, 2014. [Google Scholar] [CrossRef]
- Cao, C.; Tarlé, S.; Kaigler, D. Characterization of the Immunomodulatory Properties of Alveolar Bone-Derived Mesenchymal Stem Cells. Stem Cell Res. Ther. 2020, 11, 102. [Google Scholar] [CrossRef]
- Ledesma-Martínez, E.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem Cells Int. 2016, 2016, 4709572. [Google Scholar] [CrossRef] [PubMed]
- Ostrovidov, S.; Ramalingam, M.; Bae, H.; Orive, G.; Fujie, T.; Shi, X.; Kaji, H. Bioprinting and Biomaterials for Dental Alveolar Tissue Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 991821. [Google Scholar] [CrossRef]
- Kanjevac, T.; Gustafson, C.; Ivanovska, A.; Ravanetti, F.; Cacchioli, A.; Bosnakovski, D. Inflammatory Cytokines and Biodegradable Scaffolds in Dental Mesenchymal Stem Cells Priming. Curr. Stem Cell Res. Ther. 2019, 14, 320–326. [Google Scholar] [CrossRef]
- Ha, M.; Athirasala, A.; Tahayeri, A.; Menezes, P.P.; Bertassoni, L.E. Micropatterned Hydrogels and Cell Alignment Enhance the Odontogenic Potential of Stem Cells from Apical Papilla In-Vitro. Dent. Mater. 2020, 36, 88–96. [Google Scholar] [CrossRef]
- Ana, I.D.; Barlian, A.; Hidajah, A.C.; Wijaya, C.H.; Notobroto, H.B.; Kencana Wungu, T.D. Challenges and Strategy in Treatment with Exosomes for Cell-Free-Based Tissue Engineering in Dentistry. Future Sci. OA 2021, 7, FSO751. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Muhammad, K.; Waheed, Y. Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules 2023, 28, 6624. [Google Scholar] [CrossRef] [PubMed]
- Masoud, A.R.; Alakija, F.; Perves Bappy, M.J.; Mills, P.A.S.; Mills, D.K. Metallizing the Surface of Halloysite Nanotubes—A Review. Coatings 2023, 13, 542. [Google Scholar] [CrossRef]
- Yazdanian, M.; Rahmani, A.; Tahmasebi, E.; Tebyanian, H.; Yazdanian, A.; Mosaddad, S.A. Current and Advanced Nanomaterials in Dentistry as Regeneration Agents: An Update. Mini Rev. Med. Chem. 2021, 21, 899–918. [Google Scholar] [CrossRef]
- Nasser Atia, G.A.; Barai, H.R.; Shalaby, H.K.; Ali, N.G.; Morsy, S.M.; Ghobashy, M.M.; Nasser Attia, H.A.; Joo, S.W. Baghdadite: A Novel and Promising Calcium Silicate in Regenerative Dentistry and Medicine. ACS Omega 2022, 7, 44532–44541. [Google Scholar] [CrossRef]
- Sato, T.P.; Rodrigues, B.V.M.; Mello, D.C.R.; Münchow, E.A.; Ribeiro, J.S.; Machado, J.P.B.; Vasconcellos, L.M.R.; Lobo, A.O.; Bottino, M.C.; Borges, A.L.S. The Role of Nanohydroxyapatite on the Morphological, Physical, and Biological Properties of Chitosan Nanofibers. Clin. Oral. Investig. 2021, 25, 3095–3103. [Google Scholar] [CrossRef]
- Thurzo, A.; Gálfiová, P.; Nováková, Z.V.; Polák, Š.; Varga, I.; Strunga, M.; Urban, R.; Surovková, J.; Leško, Ľ.; Hajdúchová, Z.; et al. Fabrication and In Vitro Characterization of Novel Hydroxyapatite Scaffolds 3D Printed Using Polyvinyl Alcohol as a Thermoplastic Binder. Int. J. Mol. Sci. 2022, 23, 14870. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, A.D.; Nerantzaki, M.; Gounari, E.; Duggal, M.S.; Giannoudis, P.V.; Jha, A.; Bikiaris, D. Antibacterial Properties and Regenerative Potential of Sr2+ and Ce3+ Doped Fluorapatites; a Potential Solution for Peri-Implantitis. Sci. Rep. 2019, 9, 14469. [Google Scholar] [CrossRef]
- Iftikhar, S.; Jahanzeb, N.; Saleem, M.; ur Rehman, S.; Matinlinna, J.P.; Khan, A.S. The Trends of Dental Biomaterials Research and Future Directions: A Mapping Review. Saudi Dent. J. 2021, 33, 229–238. [Google Scholar] [CrossRef]
- Naik, S.V.; Prakash, A.J.; Prabhakar Attiguppe, R. A Survey on Awareness and Knowledge among Dentist Practicing Regenerative Endodontics towards Current Regenerative Endodontic Protocols and the Scaffolds Used in Regenerative Dentistry. Saudi Dent. J. 2023, 35, 559–566. [Google Scholar] [CrossRef]
- Guazzo, R.; Gardin, C.; Bellin, G.; Sbricoli, L.; Ferroni, L.; Ludovichetti, F.S.; Piattelli, A.; Antoniac, I.; Bressan, E.; Zavan, B. Graphene-Based Nanomaterials for Tissue Engineering in the Dental Field. Nanomaterials 2018, 8, 349. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.P.C.; Neves, J.G.; Navarro da Rocha, D.; Lopes, C.C.; Moraes, Â.M.; Correr-Sobrinho, L.; Correr, A.B. Chitosan/Xanthan/Hydroxyapatite-Graphene Oxide Porous Scaffold Associated with Mesenchymal Stem Cells for Dentin-Pulp Complex Regeneration. J. Biomater. Appl. 2023, 37, 1605–1616. [Google Scholar] [CrossRef]
- Giordano-Kelhoffer, B.; Rodríguez-Gonzalez, R.; Perpiñan-Blasco, M.; Buitrago, J.O.; Bosch, B.M.; Perez, R.A. A Novel Chitosan Composite Biomaterial with Drug Eluting Capacity for Maxillary Bone Regeneration. Materials 2023, 16, 685. [Google Scholar] [CrossRef]
- Hengtrakool, C.; Wanichpakorn, S.; Kedjarune-Leggat, U. Chitosan Resin-Modified Glass Ionomer Cement Containing Epidermal Growth Factor Promotes Pulp Cell Proliferation with a Minimum Effect on Fluoride and Aluminum Release. Polymers 2023, 15, 3511. [Google Scholar] [CrossRef] [PubMed]
- Ezeldeen, M.; Loos, J.; Mousavi Nejad, Z.; Cristaldi, M.; Murgia, D.; Braem, A.; Jacobs, R. 3D-Printing-Assisted Fabrication of Chitosan Scaffolds from Different Sources and Cross-Linkers for Dental Tissue Engineering. Eur. Cell Mater. 2021, 41, 485–501. [Google Scholar] [CrossRef]
- Shenoi, P.R.; Morey, E.S.; Makade, C.S.; Gunwal, M.K.; Khode, R.T.; Wanmali, S.S. In Vitro Evaluation of the Antimicrobial Efficacy of Chitosan and Other Endodontic Irrigants against Enterococcus Faecalis. Gen. Dent. 2016, 64, 60–63. [Google Scholar] [PubMed]
- Shrestha, A.; Kishen, A. Antibacterial Nanoparticles in Endodontics: A Review. J. Endod. 2016, 42, 1417–1426. [Google Scholar] [CrossRef]
- Ballal, N.V.; Kundabala, M.; Bhat, K.S.; Acharya, S.; Ballal, M.; Kumar, R.; Prakash, P.Y. Susceptibility of Candida Albicans and Enterococcus Faecalis to Chitosan, Chlorhexidine Gluconate and Their Combination in Vitro. Aust. Endod. J. 2009, 35, 29–33. [Google Scholar] [CrossRef]
- Kishen, A.; Shi, Z.; Shrestha, A.; Neoh, K.G. An Investigation on the Antibacterial and Antibiofilm Efficacy of Cationic Nanoparticulates for Root Canal Disinfection. J. Endod. 2008, 34, 1515–1520. [Google Scholar] [CrossRef]
- Lin, G.S.S.; Cher, C.Y.; Goh, Y.H.; Chan, D.Z.K.; Karobari, M.I.; Lai, J.C.H.; Noorani, T.Y.; Dodero, A.; Sim, G.; Lin, S.; et al. An Insight into the Role of Marine Biopolymer Alginate in Endodontics: A Review. Mar. Drugs 2022, 20, 539. [Google Scholar] [CrossRef]
- Swanson, W.B.; Mahmoud, A.H.; Woodbury, S.; Bottino, M.C. Methacrylated Gelatin as an On-Demand Injectable Vehicle for Drug Delivery in Dentistry. Methods Mol. Biol. 2023, 2588, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.G.; Bordini, E.A.F.; Bronze-Uhle, E.S.; Cassiano, F.B.; Silva, I.S.P.; Gallinari, M.O.; Matheus, H.R.; Almeida, J.M.; Cintra, L.T.A.; Hebling, J.; et al. Chitosan-Calcium-Simvastatin Scaffold as an Inductive Cell-Free Platform. J. Dent. Res. 2021, 100, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Marjanowski, S.D.; Kono, M.; Katagiri, H.; Miron, R.J.; Schaller, B. In Vitro Comparison of Macrophage Polarization and Osteoblast Differentiation Potentials between Granules and Block Forms of Deproteinized Bovine Bone Mineral. Materials 2020, 13, 2682. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Yu, F.; Xiang, J.; Zhou, K.; Zhou, L.; Zhang, Z.; Rong, X.; Ding, Z.; Wu, J.; Li, W.; et al. Mechanistically Scoping Cell-Free and Cell-Dependent Artificial Scaffolds in Rebuilding Skeletal and Dental Hard Tissues. Adv. Mater. 2022, 34, e2107922. [Google Scholar] [CrossRef] [PubMed]
- Čverha, M.; Varga, I.; Trenčanská, T.; Šufliarsky, B.; Thurzo, A. The Evolution of Robin Sequence Treatment Based on the Biomimetic Interdisciplinary Approach: A Historical Review. Biomimetics 2023, 8, 536. [Google Scholar] [CrossRef] [PubMed]
- Surovková, J.; Haluzová, S.; Strunga, M.; Urban, R.; Lifková, M.; Thurzo, A. The New Role of the Dental Assistant and Nurse in the Age of Advanced Artificial Intelligence in Telehealth Orthodontic Care with Dental Monitoring: Preliminary Report. Appl. Sci. 2023, 13, 5212. [Google Scholar] [CrossRef]
- Iacob, M.C.; Popescu, D.; Petcu, D.; Marinescu, R. Assessment of the Flexural Fatigue Performance of 3D-Printed Foot Orthoses Made from Different Thermoplastic Polyurethanes. Appl. Sci. 2023, 13, 12149. [Google Scholar] [CrossRef]
- Eck, U.; Wechner, M.; Pankratz, F.; Yu, K.; Lazarovici, M.; Navab, N. Real-Time 3D Reconstruction Pipeline for Room-Scale, Immersive, Medical Teleconsultation. Appl. Sci. 2023, 13, 10199. [Google Scholar] [CrossRef]
- Rossi, T.; Williams, A.; Sun, Z. Three-Dimensional Printed Liver Models for Surgical Planning and Intraoperative Guidance of Liver Cancer Resection: A Systematic Review. Appl. Sci. 2023, 13, 10757. [Google Scholar] [CrossRef]
- Singh, P.N.; Byram, P.K.; Das, L.; Chakravorty, N. Natural Polymer-Based Thin Film Strategies for Skin Regeneration in Lieu of Regenerative Dentistry. Tissue Eng. Part C Methods 2023, 29, 242–256. [Google Scholar] [CrossRef]
- Mohabatpour, F.; Duan, X.; Yazdanpanah, Z.; Tabil, X.L.; Lobanova, L.; Zhu, N.; Papagerakis, S.; Chen, X.; Papagerakis, P. Bioprinting of Alginate-Carboxymethyl Chitosan Scaffolds for Enamel Tissue Engineering In Vitro. Biofabrication 2022, 15, 015022. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N. Bioreactors Design, Types, Influencing Factors and Potential Application in Dentistry. A Literature Review. Curr. Stem Cell Res. Ther. 2019, 14, 351–366. [Google Scholar] [CrossRef]
- Morrison, D.G.; Tomlinson, R.E. Leveraging Advancements in Tissue Engineering for Bioprinting Dental Tissues. Bioprinting 2021, 23, e00153. [Google Scholar] [CrossRef] [PubMed]
- Osypko, K.; Ciszyński, M.; Kubasiewicz-Ross, P.; Hadzik, J. Bone Tissue 3D Bioprinting in Regenerative Dentistry through the Perspective of the Diamond Concept of Healing: A Narrative Review. Adv. Clin. Exp. Med. 2023, 32, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Diana, R.; Ardhani, R.; Kristanti, Y.; Santosa, P. Dental Pulp Stem Cells Response on the Nanotopography of Scaffold to Regenerate Dentin-Pulp Complex Tissue. Regen. Ther. 2020, 15, 243–250. [Google Scholar] [CrossRef]
- Zamudio-Ceja, R.B.; Garcia-Contreras, R.; Chavez-Granados, P.A.; Aranda-Herrera, B.; Alvarado-Garnica, H.; Jurado, C.A.; Fischer, N.G. Decellularized Scaffolds of Nopal (Opuntia Ficus-Indica) for Bioengineering in Regenerative Dentistry. J. Funct. Biomater. 2023, 14, 252. [Google Scholar] [CrossRef] [PubMed]
- Daghrery, A.; de Souza Araújo, I.J.; Castilho, M.; Malda, J.; Bottino, M.C. Unveiling the Potential of Melt Electrowriting in Regenerative Dental Medicine. Acta Biomater. 2023, 156, 88–109. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Satya Permana, M.; Winarni, I.; Van Der Heide, E. Adopted Walking Condition for Computational Simulation Approach on Bearing of Hip Joint Prosthesis: Review over the Past 30 Years. Heliyon 2022, 8, e12050. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In Silico Contact Pressure of Metal-on-Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Tauviqirrahman, M.; Ammarullah, M.I.; Jamari, J.; Saputra, E.; Winarni, T.I.; Kurniawan, F.D.; Shiddiq, S.A.; van der Heide, E. Analysis of Contact Pressure in a 3D Model of Dual-Mobility Hip Joint Prosthesis under a Gait Cycle. Sci. Rep. 2023, 13, 3564. [Google Scholar] [CrossRef]
- Fabricky, M.M.C.; Gabor, A.G.; Milutinovici, R.A.; Watz, C.G.; Avram, Ș.; Drăghici, G.; Mihali, C.V.; Moacă, E.A.; Dehelean, C.A.; Galuscan, A.; et al. Scaffold-Type Structure Dental Ceramics with Different Compositions Evaluated through Physicochemical Characteristics and Biosecurity Profiles. Materials 2021, 14, 2266. [Google Scholar] [CrossRef] [PubMed]
- Enukashvily, N.I.; Dombrovskaya, J.A.; Kotova, A.V.; Semenova, N.; Karabak, I.; Banashkov, R.E.; Baram, D.; Paderina, T.; Bilyk, S.S.; Grimm, W.D.; et al. Fibrin Glue Implants Seeded with Dental Pulp and Periodontal Ligament Stem Cells for the Repair of Periodontal Bone Defects: A Preclinical Study. Bioengineering 2021, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, W.L.; Zhang, C. Scaffold-Based and Scaffold-Free Strategies in Dental Pulp Regeneration. J. Endod. 2020, 46, S81–S89. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, B.; Palmieri, F.; Amantea, M.; Nuzzolese, M.; Valletta, R.; Zavan, B.; De Vito, D. Promising Scaffold-Free Approaches in Translational Dentistry. Int. J. Environ. Res. Public. Health 2020, 17, 3001. [Google Scholar] [CrossRef]
- Thurzo, A.; Strunga, M.; Havlínová, R.; Reháková, K.; Urban, R.; Surovková, J.; Kurilová, V. Smartphone-Based Facial Scanning as a Viable Tool for Facially Driven Orthodontics? Sensors 2022, 22, 7752. [Google Scholar] [CrossRef]
- Urban, R.; Haluzová, S.; Strunga, M.; Surovková, J.; Lifková, M.; Tomášik, J.; Thurzo, A. AI-Assisted CBCT Data Management in Modern Dental Practice: Benefits, Limitations and Innovations. Electronics 2023, 12, 1710. [Google Scholar] [CrossRef]
- Thurzo, A.; Jančovičová, V.; Hain, M.; Thurzo, M.; Novák, B.; Kosnáčová, H.; Lehotská, V.; Moravanský, N.; Varga, I. Human Remains Identification Using Micro-CT, Spectroscopic and A.I. Methods in Forensic Experimental Reconstruction of Dental Patterns After Concentrated Acid Significant Impact. Molecules 2022, 27, 4035. [Google Scholar] [CrossRef]
- Oshima, M.; Inoue, K.; Nakajima, K.; Tachikawa, T.; Yamazaki, H.; Isobe, T.; Sugawara, A.; Ogawa, M.; Tanaka, C.; Saito, M.; et al. Functional Tooth Restoration by Next-Generation Bio-Hybrid Implant as a Bio-Hybrid Artificial Organ Replacement Therapy. Sci. Rep. 2014, 4, 6044. [Google Scholar] [CrossRef]
- Wei, F.; Song, T.; Ding, G.; Xu, J.; Liu, Y.; Liu, D.; Fan, Z.; Zhang, C.; Shi, S.; Wang, S. Functional Tooth Restoration by Allogeneic Mesenchymal Stem Cell-Based Bio-Root Regeneration in Swine. Stem Cells Dev. 2013, 22, 1752. [Google Scholar] [CrossRef]
- Murashima-Suginami, A.; Takahashi, K.; Kawabata, T.; Sakata, T.; Tsukamoto, H.; Sugai, M.; Yanagita, M.; Shimizu, A.; Sakurai, T.; Slavkin, H.C.; et al. Rudiment Incisors Survive and Erupt as Supernumerary Teeth as a Result of USAG-1 Abrogation. Biochem. Biophys. Res. Commun. 2007, 359, 549–555. [Google Scholar] [CrossRef]
- Mishima, S.; Takahashi, K.; Kiso, H.; Murashima-Suginami, A.; Tokita, Y.; Jo, J.I.; Uozumi, R.; Nambu, Y.; Huang, B.; Harada, H.; et al. Local Application of Usag-1 SiRNA Can Promote Tooth Regeneration in Runx2-Deficient Mice. Sci. Rep. 2021, 11, 13674. [Google Scholar] [CrossRef]
- Takahashi, K.; Kiso, H.; Murashima-Suginami, A.; Tokita, Y.; Sugai, M.; Tabata, Y.; Bessho, K. Development of Tooth Regenerative Medicine Strategies by Controlling the Number of Teeth Using Targeted Molecular Therapy. Inflamm. Regen. 2020, 40, 21. [Google Scholar] [CrossRef]
- Murashima-Suginami, A.; Kiso, H.; Tokita, Y.; Mihara, E.; Nambu, Y.; Uozumi, R.; Tabata, Y.; Bessho, K.; Takagi, J.; Sugai, M.; et al. Anti-USAG-1 Therapy for Tooth Regeneration through Enhanced BMP Signaling. Sci. Adv. 2021, 7, eabf1798. [Google Scholar] [CrossRef] [PubMed]
- Furquim, C.P.; Kumagai, R.Y.; Bustillos-Torrez, W.; Meza-Mauricio, J.; Tanaka, C.J.; Santana, V.; Retamal-Valdes, B.; Shibli, J.A. Whole Tooth Regeneration: Can Animal Studies Be Translated into Clinical Application? Tissue Eng. Part C Methods 2022, 28, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kiso, H.; Saito, K.; Togo, Y.; Tsukamoto, H.; Huang, B.; Bessho, K. Feasibility of Gene Therapy for Tooth Regeneration by Stimulation of a Third Dentition. In Gene Therapy—Tools and Potential Applications; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Thesleff, I. From Understanding Tooth Development to Bioengineering of Teeth. Eur. J. Oral. Sci. 2018, 126 (Suppl. S1), 67–71. [Google Scholar] [CrossRef] [PubMed]
- Rokaya, D.; Skallevold, H.E.; Srimaneepong, V.; Marya, A.; Shah, P.K.; Khurshid, Z.; Zafar, M.S.; Sapkota, J. Shape Memory Polymeric Materials for Biomedical Applications: An Update. J. Compos. Sci. 2023, 7, 24. [Google Scholar] [CrossRef]
- Krasilnikova, O.; Yakimova, A.; Ivanov, S.; Atiakshin, D.; Kostin, A.A.; Sosin, D.; Shegay, P.; Kaprin, A.D.; Klabukov, I.; Makarevich, P.; et al. Gene-Activated Materials in Regenerative Dentistry: Narrative Review of Technology and Study Results. Int. J. Mol. Sci. 2023, 24, 16250. [Google Scholar] [CrossRef]
- Fakheran, O.; Fischer, K.R.; Schmidlin, P.R. Enamel Matrix Derivatives as an Adjunct to Alveolar Ridge Preservation—A Systematic Review. Dent. J. 2023, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.M.A.D.; Patano, A.; Di Pede, C.; Inchingolo, A.M.A.D.; Palmieri, G.; de Ruvo, E.; Campanelli, M.; Buongiorno, S.; Carpentiere, V.; Piras, F.; et al. Autologous Tooth Graft: Innovative Biomaterial for Bone Regeneration. Tooth Transformer® and the Role of Microbiota in Regenerative Dentistry. A Systematic Review. J. Funct. Biomater. 2023, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.M.A.D.; Latini, G.; Palmieri, G.; Di Pede, C.; Trilli, I.; Ferrante, L.; Inchingolo, A.M.A.D.; Palermo, A.; Lorusso, F.; et al. Application of Graphene Oxide in Oral Surgery: A Systematic Review. Materials 2023, 16, 6293. [Google Scholar] [CrossRef]
- Naeem, M.M.; Sarwar, H.; Nisar, A.; Ahmed, S.; Shabbir, J.; Khurshid, Z.; Palma, P.J. Effect of Propolis on Root Dentine Microhardness When Used as an Intracanal Medicament: An In Vitro Study. J. Funct. Biomater. 2023, 14, 144. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.; Min, K.-S.; Sequeira, D.B.; Diogo, P.; Gomes, B.P.F.A.; Peça, J.; Miguel, J.; Santos, M. Scaffolds for Dentin–Pulp Complex Regeneration. Medicina 2023, 60, 7. [Google Scholar] [CrossRef]
- Thurzo, A.; Urbanová, W.; Neuschlová, I.; Paouris, D.; Čverha, M. Use of Optical Scanning and 3D Printing to Fabricate Customized Appliances for Patients with Craniofacial Disorders. Semin. Orthod. 2022, 28, 92–99. [Google Scholar] [CrossRef]


| Decade | Scaffold Type | Key Features |
|---|---|---|
| Early 1990s | Natural Materials (collagen, alginate, silk, hyaluronic acid, chitosan) | Biocompatible, biodegradable |
| Late 1990s–Early 2000s | Synthetic Polymers (PLA, PGA) | Controlled pore size and architecture |
| Mid 2000s | Hybrid Scaffolds (various combinations of natural and synthetic polymers) | Biocompatibility, tunable properties |
| Late 2000s–Early 2010s | 3D Printed Scaffolds (HAp, TCP, PLA, PGLA, PCL, collagen) | Precision, complex architectures |
| Mid-2010s–Present (last decade) | Advanced materials (composites, hydrogels, bioactive materials) | Growth factors, signaling molecules, early explorations of using 4D materials 1 in scaffold development, bioactive agents, and other 2 |
| # | Authors | Title | Citations | Reference | Published |
|---|---|---|---|---|---|
| 1 | Tahriri et al. | Graphene and its derivatives: Opportunities and challenges in dentistry. | 147 | [32] | 2019 |
| 2 | Tatullo et al. | PLA-Based Mineral-Doped Scaffolds Seeded with Human Periapical Cyst-Derived MSCs: A Promising Tool for Regenerative Healing in Dentistry. | 71 | [33] | 2019 |
| 3 | Ducret et al. | Design and characterization of a chitosan-enriched fibrin hydrogel for human dental pulp regeneration. | 54 | [34] | 2019 |
| 4 | Matichescu et al. | Advanced Biomaterials and Techniques for Oral Tissue Engineering and Regeneration-A Review. | 50 | [35] | 2020 |
| 5 | Ma et al. | Three-dimensional printing biotechnology for the regeneration of the tooth and tooth-supporting tissues. | 44 | [36] | 2019 |
| 6 | Yelick et al. | Tooth Bioengineering and Regenerative Dentistry. | 43 | [37] | 2019 |
| 7 | Prahasanti et al. | Exfoliated Human Deciduous Tooth Stem Cells Incorporating Carbonate Apatite Scaffold Enhance BMP-2, BMP-7 and Attenuate MMP-8 Expression During Initial Alveolar Bone Remodeling in Wistar Rats (Rattus norvegicus). | 38 | [38] | 2020 |
| 8 | Alipour et al. | The osteogenic differentiation of human dental pulp stem cells in alginate-gelatin/Nano-hydroxyapatite microcapsules. | 36 | [39] | 2021 |
| 9 | Sukpaita et al. | Chitosan-Based Scaffold for Mineralized Tissues Regeneration. | 34 | [40] | 2021 |
| 10 | Baranova et al. | Tooth Formation: Are the Hardest Tissues of Human Body Hard to Regenerate? | 31 | [41] | 2020 |
| Material | Type | Advantage | Disadvantages | Cit. |
|---|---|---|---|---|
| Collagen | Organic | Collagen is one of the most frequently used biopolymers in the preparation of scaffolds for the regeneration of hard tissues of the oral cavity. It acts as the fundamental biological component for various tissues in the oral and craniofacial area. Its minimal immunogenicity, excellent biocompatibility, and straightforward preparation methods from diverse sources make collagen a favorable choice as a potential commercial ingredient for creating biomaterials. Collagen can be effectively modified by many chemical and physical approaches to fabricate scaffolds in different forms (e.g., membranes, sponges, gels). Furthermore, incorporating inorganic elements like hydroxyapatite (HAp) and β-tricalcium phosphate (β-TCP) through hybridization can result in the development of mineralized collagen scaffolds. This enhances the scaffolds’ mechanical properties, biodegradability, and ability to induce osteogenesis. | Collagen scaffolds derived from natural sources through freeze-drying or electrospinning exhibit insufficient mechanical strength and biostability. This inadequacy has prompted persistent endeavors to enhance these scaffolds through physical, chemical, and biological modifications. | [42,43,44,45] |
| Gelatin | Organic | Gelatin, as a hydrophilic polymer, exhibits exceptional sol–gel transition characteristics and biocompatibility, rendering it a versatile material within the realm of hydrogels. Utilizing gelatin as a matrix for hydrogels enables the replication of diverse tissue characteristics and facilitates the customization of hydrogel properties, including mechanics and degradation. This adaptability makes it well-suited for a broad spectrum of biomedical applications. Studies have shown that the dental light-curing process of gelatin can sustain the viability of adult dentin cells, highlighting its potential application in the field of dentistry. Furthermore, experiments conducted in vitro demonstrated the noteworthy bioactivity of the hydrogels, as they effectively preserved the chondrocyte phenotype while fostering cell adhesion and proliferation. | Gelatin is characterized by insufficient mechanical strength. It is not suitable for applications that demand advanced adjustability in terms of cell adhesion, migration, and degradation mediated by cells. | [46,47,48] |
| Chitosan | Organic | Chitosan, a natural biomaterial primarily derived from chitin, possesses several advantageous characteristics, including biocompatibility, hydrophilicity, biodegradability, and a wide-ranging antibacterial spectrum that encompasses both Gram-negative and Gram-positive bacteria, as well as fungi. Furthermore, its molecular structure features reactive functional groups, offering numerous sites for reactions and opportunities to establish electrochemical connections at the cellular and molecular levels. Chitosan support cell proliferation and cellular activity of osteoblasts and chondrocytes. In addition, research efforts have extensively explored composite formulations involving chitosan and hydroxyapatite, aiming to create templates of chitosan and hydroxyapatite through innovative methodologies. | Chitosan’s limitations in the regeneration of hard tissues in the oral cavity include challenges such as its mechanical properties, potential degradation issues, and the need for further research to optimize its effectiveness in this specific application. | [49,50,51,52] |
| Polylactic-co-glycolic acid (PLGA) | Organic polymer | PLGA is generally considered to be a biocompatible material, meaning that it is well-tolerated by the body. PLGA is a biodegradable material, meaning that it breaks down over time into naturally occurring metabolites. This property makes it suitable for applications where the material needs to be eliminated from the body over time. PLGA has good mechanical properties, making it suitable for a wide range of applications. For example, PLGA is used to make surgical sutures that need to be strong enough to hold a wound together, but also flexible enough to not break.The rate of degradation of PLGA depends on the ratio of L-lactic acid to glycolic acid in the copolymer. Copolymers with a higher content of L-lactic acid degrade more slowly than copolymers with a higher content of glycolic acid. This property can be an advantage in some applications, such as the production of implants that need to last for a long time. | The degradation rate of PLGA depends on the ratio of L-lactic acid to glycolic acid in the copolymer. Copolymers with a higher content of L-lactic acid degrade more slowly than copolymers with a higher content of glycolic acid. This property can be a disadvantage in some applications, such as the production of implants that need to last for a long time. PLGA is more expensive than some other materials used in medicine. In some cases, PLGA toxicity can occur, usually caused by the L-lactic acid monomer. PLGA toxicity can be particularly problematic in applications where the material is in contact with blood or other body fluids. In some cases, allergic reactions to PLGA can occur. These reactions are usually mild and go away on their own, but sometimes can be severe and even fatal. | [53,54,55,56] |
| Polycaprolactone (PCL) | Organic polymer | PCL is generally considered to be a biocompatible material, meaning that it is well-tolerated by the body. PCL also promotes a very good biodegradability, meaning that it breaks down over time into the natural metabolites. This is an advantage for applications where material needs to be eliminated from the body over time. PCL has good mechanical properties, making it suitable for a wide range of applications.: PCL is easily moldable and processable, making it easy to use in medical applications. | The rate of degradation of PCL depends on the ratio of caprolactone to other monomers used in its production. Copolymers with a higher content of caprolactone degrade more slowly than copolymers with a lower content of caprolactone. This property can be a problem in some applications, such as implants that need to last for a long time. PCL is more expensive than some other materials used in medicine. In some cases, allergic reactions to PCL can occur. | [53,57,58] |
| Alginates | Organic | Alginates are generally considered to be biocompatible materials, meaning that they are well-tolerated by the body. This property makes them suitable for applications where the material needs to be in contact with human tissue. They are also biodegradable, and can break down into naturally occurring metabolites. This property makes them suitable for applications where the material needs to be eliminated from the body over time.Alginates have good mechanical properties, making them suitable for applications where the material needs to be strong enough to perform its function. Alginates also presents good liquid absorption, making them suitable for application where material needs to absorb fluids from the body. Alginates have antibacterial properties, making them suitable for applications where it is necessary to prevent infection. | The degradation rate of alginates depends on the ratio of mannuronic acid to guluronic acid in the polysaccharide. Alginates with a higher content of mannuronic acid degrade faster than alginates with a higher content of guluronic acid. This property can be a disadvantage in some applications, such as the production of implants that need to last for a long time. Alginates are more expensive than some other materials used in medicine. In some cases, allergic reactions to alginates can occur. | [59,60,61] |
| Hyaluronic acid (HA) | Organic | HA is a linear, hydrophilic, polyanionic polysaccharide, and is a natural biological component of living organisms. It has good bioactivity, biocompatibility, and biodegradability, in the human body. The HA has multiple physiological roles, including water regulation in tissue matrices, skin wound regeneration processes, cartilage resistance to compression, act as joint lubricant and shock absorber, etc. For regenerative medicine, HA can be used as a reservoir of stimulants such as growth factors, etc. | HA properties are affected by structural and chemical complexity depending on its molecular weight, it has low mechanical strength, and may induce immunoreactivity, e.g., granulomatous foreign body reaction. | [62,63,64,65,66,67,68] |
| Bioactive glasses | Inorganic | Bioactive glass has potential for dental applications, such as dentin regeneration, due to its excellent bioactivity, and easy enhancement of functionality by specific therapeutic ions doping with, e.g., antibacterial and angiogenetic behavior. It has an excellent ability to bond with both hard and soft tissues. | Limited applications for low level loading replacements due to its low mechanical strength and brittleness. The processing challenges and the costs, in certain cases also slow degradation may be an issue. | [69,70,71,72,73] |
| hydroxyapatite (HAp) | Inorganic | HAp is a natural component of human bones and teeth. It has excellent biocompatibility and can provide stimuli for osteoinductivity and osteoconductivity. Is often used in dental applications due to its similarity to the mineral composition of natural teeth, and integrates well with the surrounding tissue. | HAp is brittle and has very low fracture toughness. Its application is complicated with difficulty in shaping. Pure HAp may have poor adhesion to soft tissues and slow integration or resorption rates. The cost of medical grade HAp are high. | [19,74,75,76,77,78] |
| Tricalcium phosphate (TCP) | Inorganic | Unlike Hap, the β-TCP bioceramics show higher solubility and biodegradation rate by osteoclast cells, which provoke a local acidification that leads to material dissolution. Osteoclasts then initiate bone resorption by releasing protons and enzymes. This process of bone resorption caused by osteoclasts is coupled with ossification of osteoblasts. By testing β-TCP ceramics, they proved their ability to support differentiation and proliferation of osteoblasts and mesenchymal cells. It has been reported to have excellent biocompatibility and osteoconductivity as well. | Lower mechanical strength as HAp ceramics. | [79,80,81,82,83] |
| Biphasic calcium phosphate (BCP) | Inorganic | Biphasic calcium phosphate has been developed as a compromise to get good mechanical properties of HAp and higher solubility and osteoconductivity of β-TCP. It is considered the gold standard of bone substitutes in bone reconstructive surgery. The advantage of BCP is the preservation of the mechanical strength during its resorption. The higher the ratio, the greater the resorbability. BCP-A (contains high amount of Calcium-deficient hydroxyapatite CDHA) significantly decreased the inflammation response of dental pulp and promotes the formation of dentin bridges. The BCP with composition of 15% HAp and 85% β-TCP forms the bone earlier and in more quantity than second-investigated BCP with composition of 85% HAp and 15% β-TCP in mandible bone of beagle dogs after 4, 12, and 26 weeks. | The ratio β-TCP/HAp should be individually tuned according to application (depending on the solubility—increased solubility of bio ceramics does not mean that resorption activity is optimal). | [84,85,86,87,88,89] |
| Calcium Phosphate Cements (CPCs): | InorganicTypical CPCs 1 | Paste—set in situ to fill bone defects. CPCs have shown potential in dental applications for filling cavities, repairing defects, and promoting bone regeneration. The main characteristic and advantage of CPCs is their injectability and/or moldability to fill optimally irregular bone defects. They form intimate contact with the bone structure ensuring good transformation into new bone. The mechanical properties, as well as setting time, varies depending on the chemical composition of CPCs. New kinds of CPCs can reach high compressive strength up to 35 MPa and setting time of 14 min. | The dense structure of CPCs lacking the microporosity that is necessary for bone ingrowth together with the slow biodegradation represent their main disadvantages. | [90,91,92,93,94,95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gašparovič, M.; Jungová, P.; Tomášik, J.; Mriňáková, B.; Hirjak, D.; Timková, S.; Danišovič, Ľ.; Janek, M.; Bača, Ľ.; Peciar, P.; et al. Evolving Strategies and Materials for Scaffold Development in Regenerative Dentistry. Appl. Sci. 2024, 14, 2270. https://doi.org/10.3390/app14062270
Gašparovič M, Jungová P, Tomášik J, Mriňáková B, Hirjak D, Timková S, Danišovič Ľ, Janek M, Bača Ľ, Peciar P, et al. Evolving Strategies and Materials for Scaffold Development in Regenerative Dentistry. Applied Sciences. 2024; 14(6):2270. https://doi.org/10.3390/app14062270
Chicago/Turabian StyleGašparovič, Michal, Petra Jungová, Juraj Tomášik, Bela Mriňáková, Dušan Hirjak, Silvia Timková, Ľuboš Danišovič, Marián Janek, Ľuboš Bača, Peter Peciar, and et al. 2024. "Evolving Strategies and Materials for Scaffold Development in Regenerative Dentistry" Applied Sciences 14, no. 6: 2270. https://doi.org/10.3390/app14062270
APA StyleGašparovič, M., Jungová, P., Tomášik, J., Mriňáková, B., Hirjak, D., Timková, S., Danišovič, Ľ., Janek, M., Bača, Ľ., Peciar, P., & Thurzo, A. (2024). Evolving Strategies and Materials for Scaffold Development in Regenerative Dentistry. Applied Sciences, 14(6), 2270. https://doi.org/10.3390/app14062270