N-Vinylcarbazole: As an Additive for Thermal Polymerization at Room Temperature with in situ Formation of Ag(0) Nanoparticules
Abstract
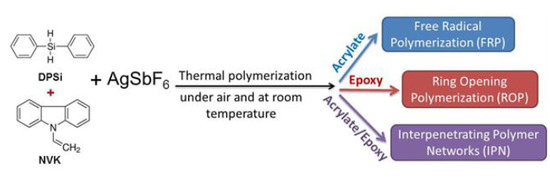
:1. Introduction



2. Experimental Section
2.1. Compounds
2.2. Radical and Cationic Polymerization
2.3. Interpenetrated Polymer Network Synthesis
2.4. Characterization
3. Results and Discussion
3.1. Free Radical Polymerization (FRP)




3.2. Cationic Polymerization (CP)

3.3. DPSi/NVK/AgSbF6 for the Synthesis of Interpenetrated Polymer Networks (IPNs)


3.4. In-situ Incorporation of Silver Nanoparticules (Ag(0)NPs)

3.5. Reaction Mechanisms

3.6. Characterization of Ag(0) Nanoparticles (NPs) and Polymer Films



4. Conclusions
Author Contributions
Conflicts of Interest
References
- Carotenuto, G.; Martorana, B.; Perlo, P.B.; Nicolais, L. A universal method for the synthesis of metal and metal sulfide clusters embedded in polymer matrices. J. Mater. Chem. 2003, 13, 2927–2930. [Google Scholar] [CrossRef]
- Biswas, A.; Aktas, O.C.; Schurmann, U.; Saeed, U.; Zaporojtchenko, V.; Faupel, F. Controlled syntheses of Ag-polytetrafluoroethylene nanocomposite thin films by co-sputtering from two magnetron sources. Appl. Phys. Lett. 2004, 84, 2655–2657. [Google Scholar]
- Sih, B.C.; Wolf, M.O. Metal nanoparticle—Conjugated polymer nanocomposites. Chem. Commun. 2005, 27, 3375–3384. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.R.F.; Bard, A.J. Chemical, electrochemical, gravimetric, and microscopic studies on antimicrobial silver films. J. Phys. Chem. B 2002, 106, 279–287. [Google Scholar] [CrossRef]
- Ouyang, J.Y.; Chu, C.W.; Szmanda, C.R.; Ma, L.P.; Yang, Y. Programmable polymer thin film and non-volatile memory device. Nat. Mater. 2004, 3, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.T.; Yen, C.C. Studies on the preparation and properties of conductive polymers. VIII. Use of heat treatment to prepare metallized films from silver chelate of PVA and PAN. J. Appl. Polym. Sci. 1995, 55, 371–374. [Google Scholar] [CrossRef]
- Shanmugam, S.; Viswanathan, B.; Varadarajan, T.K. A novel single step chemical route for noble metal nanoparticles embedded. Mater. Chem. Phys. 2006, 95, 51–55. [Google Scholar] [CrossRef]
- Lin, W.C.; Yang, M.C. Novel Silver/Poly(vinyl alcohol) Nanocomposites for Surface-Enhanced Raman Scattering-Active Substrates. Macromol. Rapid Commun. 2005, 26, 1942–1947. [Google Scholar] [CrossRef]
- Balan, L.; Burget, D. Synthesis of metal/polymer nanocomposite by UV-radiation curing. Eur. Polym. J. 2006, 42, 3180–3189. [Google Scholar] [CrossRef]
- Balan, L.; Schneider, R.; Lougnot, D.J. A new and convenient route to polyacrylate/silver nanocomposites by light-induced cross-linking polymerization. Prog. Org. Coat. 2008, 62, 351–357. [Google Scholar] [CrossRef]
- Selvan, S.T.; Spatz, J.P.; Klok, H.A.; Möller, M. Gold–Polypyrrole Core–Shell Particles in Diblock Copolymer Micelles. Adv. Mater. 1998, 10, 132–134. [Google Scholar] [CrossRef]
- Corbierre, M.K.; Cameron, N.S.; Sutton, M.; Mochrie, S.G.; Lurio, L.B.; Rühm, A.; Lennox, R.B. Polymer-Stabilized Gold Nanoparticles and Their Incorporation into Polymer Matrices. J. Am. Chem. Soc. 2001, 123, 10411–10412. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K.; Gnanou, Y.; Leibler, L. Macromolecular Engineering: From Precise Macromolecular Synthesis to Macroscopic Materials Properties and Applications; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Pappas, S.P. UV Curing: Science and Technology; Technology Marketing Corp: Aachen, Germany, 1985. [Google Scholar]
- Dietliker, K. A Compilation of Photoinitiators Commercially Available for UV Today; Sita Technology Ltd.: Edinburgh, UK; London, UK, 2002. [Google Scholar]
- Crivello, J.V. Photoinitiators for Free Radical, Cationic and Anionic Photopolymerization, 2nd ed.; Bradley, G., Ed.; Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
- Crivello, J.V. Ring-Opening Polymerization; Brunelle, D.J., Ed.; Hanser: Munich, Germany, 1993. [Google Scholar]
- Sangermano, M.; Crivello, J.V. Photoinitiated Polymerization; Belfied, K.D., Crivello, J.V., Eds.; ACS Symposium Series 847; Washington, DC, USA, 2003. [Google Scholar]
- Fouassier, J.P. Photoinitiation Photopolymerization and Photocuring: Fundamental and Applications; Hanser Publishers: New York, NY, USA, 1995. [Google Scholar]
- Radiation Curing in Polymer Science and Technology; Fouassier, J.P.; Rabek, J.F. (Eds.) Elsevier Science Publishers Ltd.: London, UK, 1993.
- El-Roz, M.; Lalevée, J.; Allonas, X.; Fouassier, J.P. Mechanistic Investigation of the Silane, Germane, and Stannane Behavior When Incorporated in Type I and Type II Photoinitiators of Polymerization in Aerated Media. Macromolecules 2009, 42, 8725–8732. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Kim, K.-J. Photoinitiated polymerization of 1,6-hexanediol diacrylate-amine synergists. J. Rad. Curing 1985, 12, 9–15. [Google Scholar]
- Decker, C.; Jenkins, A.D. Kinetic approach of oxygen inhibition in ultraviolet- and laser-induced polymerizations. Macromolecules 1985, 18, 1241–1244. [Google Scholar] [CrossRef]
- Gou, L.; Opheim, B.; Scranton, A.B. Photochemistry and UV Curing: New Trends; Fouassier, J.P., Ed.; Research Signpost: Trivandrum, India, 2006. [Google Scholar]
- Crivello, J.V.; Lam, J.H.W. Redox cationic polymerization: The diaryliodonium salt/ascorbate redox couple. J. Polym. Sci. Part A: Polym. Chem. 1981, 19, 539–548. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lee, J. Redox initiators for cationic polymerization: The diaryliodonium Salt/Sn(II) redox couple. Makromol. Chem. 1983, 184, 463–473. [Google Scholar] [CrossRef]
- Onen, A.; Yagci, Y. Redox-initiated cationic polymerization: The pyridinium salt/ascorbate redox couple. Polymer 1997, 38, 1423–1425. [Google Scholar] [CrossRef]
- Onen, A.; Denizligil, S.; Yagci, Y. Cationic polymerization using allyl sulfonium salt. 3. Allyl sulfonium salt/ascorbate redox couple. Angew. Makromol. Chem. 1997, 245, 149–154. [Google Scholar] [CrossRef]
- Crivello, J.V. Redox initiated cationic polymerization. J. Polym. Sci. Part A: Polym. Chem. 2009, 47, 1825–1835. [Google Scholar] [CrossRef]
- Souane, R.; Tehfe, M.-A.; Lalevée, J.; Gigmes, D.; Fouassier, J.-P. New Initiating Systems for Thermal Cationic Polymerization at Ambient Temperature with in situ Formation of Ag(0) Nanoparticles: A Silane/Silver Salt Combination. Macromol. Chem. Phys. 2010, 211, 1441–1445. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Jamois, R.; Cousin, P.; Elkoun, S.; Robert, M. In situ synthesis and characterization of silver/polymer nanocomposites by thermal cationic polymerization processes at room temperature: Initiating systems based on organosilanes and starch nanocrystals. Langmuir 2015, 31, 4305–4315. [Google Scholar] [CrossRef] [PubMed]
- Yagci, Y.; Sahin, O.; Ozturk, T.; Marchi, S.; Grassini, S.; Sangermano, M. Synthesis of silver/epoxy nanocomposites by visible light sensitization using highly conjugated thiophene derivatives. React. Funct. Polym. 2011, 71, 857–862. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.A.; Morlet-Savary, F.; Allonas, X.; Graff, B.; Fouassier, J.P. Oxygen mediated and wavelength tunable cationic photopolymerization reactions under air and low intensity: A new concept. Prog. Org. Coat. 2011, 70, 23–31. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Morlet-Savary, F.; Fouassier, J.P. Green Bulb Light Source Induced Epoxy Cationic Polymerization under Air Using Tris(2,20-bipyridine)ruthenium(II) and Silyl Radicals. Macromolecules 2010, 43, 10191–10195. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Peter, M.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P. Efficient dual radical/cationic photoinitiator under visible light: A new concept. Polym. Chem. 2011, 2, 1986–1991. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Peter, M.; Morlet-Savary, F.; Fouassier, J.P. A Novel Photopolymerization Initiating System Based on an Iridium Complex Photocatalyst. Macromol. Rapid Commun. 2011, 32, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, J.; Blanchard, N.; Tehfe, M.A.; Peter, M.; Morlet-Savary, F.; Fouassier, J.P. Household LED irradiation under air: Cationic polymerization using iridium or ruthenium complex photocatalysts. Polym. Bull. 2011, 68, 341–347. [Google Scholar] [CrossRef]
- Durmaz, Y.Y.; Moszner, N.; Yagci, Y. Visible Light Initiated Free Radical Promoted Cationic Polymerization Using Acylgermane Based Photoinitiator in the Presence of Onium Salts. Macromolecules 2008, 41, 6714. [Google Scholar] [CrossRef]
- Crivello, J.V. A new visible light sensitive photoinitiator system for the cationic polymerization of epoxides. J. Polym. Sci. Part A: Polym. Chem. 2009, 47, 866–875. [Google Scholar] [CrossRef]
- Crivello, J.V. Radical-Promoted Visible Light Photoinitiated Cationic Polymerization of Epoxides. J. Macromol. Sci. Part A 2009, 46, 474–483. [Google Scholar] [CrossRef]
- Tunc, D.; Yagci, Y. Thioxanthone-ethylcarbazole as a soluble visible light photoinitiator for free radical and free radical promoted cationic polymerizations. Polym. Chem. 2011, 2, 2557–2563. [Google Scholar] [CrossRef]
- Yilmaz, G.; Beyazit, S.; Yagci, Y. Visible light induced free radical promoted cationic polymerization using thioxanthone derivatives. J. Polym. Sci. Part A: Polym. Chem. 2011, 49, 1591–1596. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; El-Roz, M.; Graff, B.; Allonas, X.; Fouassier, J.P. New Photoinitiators Based on the Silyl Radical Chemistry: Polymerization Ability, ESR Spin Trapping, and Laser Flash Photolysis Investigation. Macromolecules 2008, 41, 4180–4186. [Google Scholar] [CrossRef]
- Lalevée, J.; El-Roz, M.; Allonas, X.; Fouassier, J.P. Free-radical-promoted cationic photopolymerization under visible light in aerated media: New and highly efficient silane-containing initiating systems. J. Polym. Sci. Part A: Polym. Chem. 2008, 46, 2008–2014. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Lalevée, J.; Allonas, X.; Fouassier, J.P. Long Wavelength Cationic Photopolymerization in Aerated Media: A Remarkable Titanocene/Tris(trimethylsilyl)silane/Onium Salt Photoinitiating System. Macromolecules 2009, 42, 8669–8674. [Google Scholar] [CrossRef]
- Uygun, M.; Kahveci, M.U.; Odaci, D.; Timur, S.; Yagci, Y. Antibacterial Acrylamide Hydrogels Containing Silver Nanoparticles by Simultaneous Photoinduced Free Radical Polymerization and Electron Transfer Processes. Macromol. Chem. Phys. 2009, 210, 1867–1875. [Google Scholar] [CrossRef]
- Dawson-Andoh, B.; Matuana, L. High density polyethylene-wood flour composite lumber: Efficacy of two proprietary biocides in the control of fungal colonization and discoloration. Eur. J. Wood Wood Products 2007, 65, 331–334. [Google Scholar] [CrossRef]
- Crivello, J.V. Redox initiated cationic polymerization: Reduction of diaryliodonium salts by 9-BBN. J. Polym. Sci. Part A: Polym. Chem. 2009, 47, 5639–5651. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Telitel, S.; Gigmes, D.; Contal, E.; Bertin, D.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Zinc-based metal complexes as new photocatalysts in polymerization initiating systems. Eur. Polym. J. 2013, 49, 1040–1049. [Google Scholar] [CrossRef]
- Kunioka, M.; Wang, Y.; Onozawa, S-Y. Polymerization of Poly(ε-caprolactone) Using Yttrium Triflate. Polym. J. 2003, 35, 422–429. [Google Scholar] [CrossRef]
- Lalevée, J.; Dirani, A.; El Roz, M.; Graff, B.; Allonas, X.; Fouassier, J.P. Silanes as New Highly Efficient Co-initiators for Radical Polymerization in Aerated Media. Macromolecules 2008, 41, 2003–2010. [Google Scholar] [CrossRef]
- Taleb, A.; Petit, C.; Pileni, M.P. Optical Properties of Self-Assembled 2D and 3D Superlattices of Silver Nanoparticles. J. Phys. Chem. B 1998, 102, 2214–2220. [Google Scholar] [CrossRef]
- He, S.; Yao, J.; Jiang, P.; Shi, D.; Zhang, H.; Xie, S.; Pang, S.; Gao, H. Formation of Silver Nanoparticles and Self-Assembled Two-Dimensional Ordered Superlattice. Langmuir 2001, 17, 1571–1575. [Google Scholar] [CrossRef]
- Eaborn, C. The reaction between trisubstituted silanes and silver perchlorate. Organosilicon Compounds. XIV. J. Chem. Soc. 1955, 3, 2517–2519. [Google Scholar]
- Marciniec, B. Mechanism of competitive-consecutive reaction of trisubstituted silanes with silver perchlorate in benzene. Z. Anorg. Allg. Chem. 1982, 495, 232–240. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Marks, D.L.; Miller, D.; Kesatie, D. Oxidation of silanes. III. Reaction of aryldimethylsilanes with mercuric acetate and thallium triacetate. J. Org. Chem. 1969, 34, 1769–1771. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Marks, D.L. Oxidation of silanes II. The reaction of ozone with aryldimethylsilanes. J. Organomet. Chem. 1968, 11, 407–413. [Google Scholar] [CrossRef]
- Lambert, J.B.; Kania, L.; Zhang, S. Modern Approaches to Silylium Cations in Condensed Phase. Chem. Rev. 1995, 95, 1191–1201. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.-A.; Zein-Fakih, A.; Ball, B.; Telitel, S.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P. N-Vinylcarbazole: An Additive for Free Radical Promoted Cationic Polymerization upon Visible Light. ACS Macro Lett. 2012, 1, 802–806. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Faury, T.; Graff, B.; Tehfe, M.-A.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. New core-pyrene π structure organophotocatalysts usable as highly efficient photoinitiators. Beilstein J. Org. Chem. 2013, 9, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, J.; Dumur, F.; Mayer, C.-R.; Gigmes, D.; Nasr, G.; Tehfe, M.-A.; Telitel, S.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P. Photopolymerization of N-Vinylcarbazole Using Visible-Light Harvesting Iridium Complexes as Photoinitiators. Macromolecules 2012, 45, 4134–4141. [Google Scholar] [CrossRef]
- Mokbel, H.; Dumur, F.; Telitel, S.; Vidal, L.; Xiao, P.; Versace, D.-L.; Tehfe, M.-A.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; et al. Photoinitiating systems of polymerization and in situ incorporation of metal nanoparticles into polymer matrices upon exposure to visible light: Push–pull malonate and malononitrile based dyes. Polym. Chem. 2013, 4, 5679–5687. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Graff, B.; Allonas, X.; Fouassier, J.P. Tris(trimethylsilyl)silyl versus tris(trimethylsilyl)germyl: Radical reactivity and oxidation ability. J. Organomet. Chem. 2008, 693, 3643–3649. [Google Scholar] [CrossRef]
- Zaborovskiy, A.B.; Lutsyk, D.S.; Prystansky, R.E.; Kopylets, V.I.; Timokhin, V.I.; Chatgilialoglu, C. A mechanistic investigation of (Me3Si)3SiH oxidation. J. Organomet. Chem. 2004, 689, 2912–2919. [Google Scholar] [CrossRef]
- Hua, Y.; Crivello, J.V. Synergistic interaction of epoxides and N-vinylcarbazole during photoinitiated cationic polymerization. J. Polym. Sci. A: Polym. Chem. 2000, 38, 3697–3709. [Google Scholar] [CrossRef]
- Abdoul-Rasoul, F.A.M.; Ledwith, A.; Yagci, Y. Photochemical and thermal cationic polymerizations promoted by free radical initiators. Polymer 1978, 19, 1219–1222. [Google Scholar] [CrossRef]
- Xiao, P.; Lalevée, J.; Zhao, J.; Stenzel, M.H. N-Vinylcarbazole as Versatile Photoinaddimer of Photopolymerization under Household UV LED Bulb (392 nm). Macromol. Rapid Commun. 2015. [Google Scholar] [CrossRef] [PubMed]
- Scaiano, J.C.; Billone, P.; Gonzalez, C.M.; Maretti, L.; Marin, M.L.; McGilvray, K.L.; Yuan, N. Photochemical routes to silver and gold nanoparticles. Pure Appl. Chem. 2009, 81, 635–647. [Google Scholar] [CrossRef]
- McGilvray, K.L.; Decan, M.R.; Wang, D.; Scaiano, J.C. Facile Photochemical Synthesis of Unprotected Aqueous Gold Nanoparticles. J. Am. Chem. Soc. 2006, 128, 15980–15981. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.L.; McGilvray, K.L.; Scaiano, J.C. Photochemical Strategies for the Synthesis of Gold Nanoparticles from Au(III) and Au(I) Using Photoinduced Free Radical Generation. J. Am. Chem. Soc. 2008, 130, 16572–16584. [Google Scholar] [CrossRef] [PubMed]
- Stamplecoskie, K.G.; Scaiano, J.C. Kinetics of the Formation of Silver Dimers: Early Stages in the Formation of Silver Nanoparticles. J. Am. Chem. Soc. 2011, 133, 3913–3920. [Google Scholar] [CrossRef] [PubMed]
- Sangermano, M.; Yagci, Y.; Rizza, G. In Situ Synthesis of Silver-Epoxy Nanocomposites by Photoinduced Electron Transfer and Cationic Polymerization Processes. Macromolecules 2007, 40, 8827–8829. [Google Scholar] [CrossRef]
- Yagci, Y.; Sangermano, M.; Rizza, G. A visible light photochemical route to silver-epoxy nanocomposites by simultaneous polymerization–reduction approach. Polymer 2008, 49, 5195–5198. [Google Scholar] [CrossRef]
- Yagci, Y.; Sangermano, M.; Rizza, G. In situ synthesis of gold-cross-linked poly(ethylene glycol) nanocomposites by photoinduced electron transfer and free radical polymerization processes. Chem. Commun. 2008, 24, 2771–2773. [Google Scholar]
- Nehlig, E.; Schneider, R.; Vidal, L.; Clavier, G.; Balan, L. Silver Nanoparticles Coated with Thioxanthone Derivative as Hybrid Photoinitiating Systems for Free Radical Polymerization. Langmuir 2012, 28, 17795–17802. [Google Scholar] [CrossRef] [PubMed]
- Buruiana, T.; Melinte, V.; Jitaru, F.; Buruiana, E.-C.; Balan, L. Preparation of siloxane-based urethane dimethacrylates carrying carboxylic groups and the effect of silver nanoparticles on the properties of composite polymer films. Polym. Chem. Part A 2012, 50, 874–883. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tehfe, M.-A.; Elkoun, S.; Robert, M. N-Vinylcarbazole: As an Additive for Thermal Polymerization at Room Temperature with in situ Formation of Ag(0) Nanoparticules. Appl. Sci. 2015, 5, 241-258. https://doi.org/10.3390/app5030241
Tehfe M-A, Elkoun S, Robert M. N-Vinylcarbazole: As an Additive for Thermal Polymerization at Room Temperature with in situ Formation of Ag(0) Nanoparticules. Applied Sciences. 2015; 5(3):241-258. https://doi.org/10.3390/app5030241
Chicago/Turabian StyleTehfe, Mohamad-Ali, Saïd Elkoun, and Mathieu Robert. 2015. "N-Vinylcarbazole: As an Additive for Thermal Polymerization at Room Temperature with in situ Formation of Ag(0) Nanoparticules" Applied Sciences 5, no. 3: 241-258. https://doi.org/10.3390/app5030241
APA StyleTehfe, M. -A., Elkoun, S., & Robert, M. (2015). N-Vinylcarbazole: As an Additive for Thermal Polymerization at Room Temperature with in situ Formation of Ag(0) Nanoparticules. Applied Sciences, 5(3), 241-258. https://doi.org/10.3390/app5030241