Synthetic Applications of the Parkins Nitrile Hydration Catalyst [PtH{(PMe2O)2H}(PMe2OH)]: A Review
Abstract
:1. Introduction


2. Preparation of Complex [PtH{(PMe2O)2H}(PMe2OH)], First Catalytic Studies and Mechanism of Action


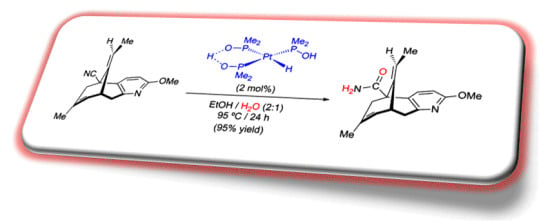
3. Application of Complex [PtH{(PMe2O)2H}(PMe2OH)] in the Total Synthesis of Natural Products













4. Application of Complex [PtH{(PMe2O)2H}(PMe2OH)] in the Synthesis of Other Biologically Active Molecules and Pharmaceutical Compounds





5. Other Synthetic Applications of Complex [PtH{(PMe2O)2H}(PMe2OH)]





6. Limitations



7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Beller, M.; Bolm, C. Transition Metals for Organic Synthesis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Hegedus, L.S.; Söderberg, B.C.G. Transition Metals in the Synthesis of Complex Organic Molecules, 3rd ed.; University Science Books: Sausalito, CA, USA, 2009. [Google Scholar]
- Crawley, M.L.; Trost, B.M. Applications of Transition Metal Catalysts in Drug Discovery and Development; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Zabicky, J. The Chemistry of Amides; John Wiley & Sons: New York, NY, USA, 1970. [Google Scholar]
- Greenberg, A.; Breneman, C.M.; Liebman, J.F. The Amide Linkage: Structural Significance in Chemistry, Biochemistry and Materials Science; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Lanigan, R.M.; Sheppard, T.D. Recent developments in amide synthesis: Direct amidation of carboxylic acids and transamidation reactions. Eur. J. Org. Chem. 2013, 2013, 7453–7465. [Google Scholar] [CrossRef]
- Bailey, P.D.; Mills, T.J.; Pettecrew, R.; Price, R.A. Amides. In Comprehensive Organic Functional Group Transformations, 2nd ed.; Katritzky, A.R., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2005; Volume 5, pp. 201–294. [Google Scholar]
- Dopp, D.; Dopp, H. Methoden Organic Chemistry (Houben Weyl); Thieme Verlag: Stuttgart, Germany, 1985; Volume E5(2), pp. 1024–1031. [Google Scholar]
- Parkins, A.W. Catalytic hydration of nitriles to amides: Platinum containing catalyst offers new opportunity. Platinum Metals Rev. 1996, 40, 169–174. [Google Scholar]
- Kukushkin, V.Y.; Pombeiro, A.J.L. Additions to metal-activated organonitriles. Chem. Rev. 2002, 102, 1771–1802. [Google Scholar] [CrossRef] [PubMed]
- Kukushkin, V.Y.; Pombeiro, A.J.L. Metal-mediated and metal-catalyzed hydrolysis of nitriles. Inorg. Chim. Acta 2005, 358, 1–21. [Google Scholar] [CrossRef]
- Ahmed, T.J.; Knapp, S.M.M.; Tyler, D.R. Frontiers in catalytic nitrile hydration: Nitrile and cyanohydrins hydration catalyzed by homogeneous organometallic complexes. Coord. Chem. Rev. 2011, 255, 949–974. [Google Scholar] [CrossRef]
- García-Álvarez, R.; Crochet, P.; Cadierno, V. Metal-catalyzed amide bond forming reactions in an environmentally friendly aqueous medium: Nitrile hydrations and beyond. Green Chem. 2013, 15, 46–66. [Google Scholar] [CrossRef]
- García-Álvarez, R.; Francos, J.; Tomás-Mendivil, E.; Crochet, P.; Cadierno, V. Metal-catalyzed nitrile hydration reactions: The specific contribution of ruthenium. J. Organomet. Chem. 2014, 771, 93–104. [Google Scholar] [CrossRef]
- Downs, E.L.; Tyler, D.R. Nanoparticle catalysts for nitrile hydration. Coord. Chem. Rev. 2014, 280, 28–37. [Google Scholar] [CrossRef]
- Ghaffar, T.; Parkins, A.W. A new homogeneous platinum containing catalyst for the hydrolysis of nitriles. Tetrahedron Lett. 1995, 36, 8657–8660. [Google Scholar] [CrossRef]
- Parkins, A.W.; Ghaffar, T. Catalyst and process for preparing amides. PCT Int. Appl. WO96/30379, 1996. [Google Scholar]
- Ghaffar, T.; Parkins, A.W. The catalytic hydration of nitriles to amides using a homogeneous platinum phosphinito catalyst. J. Mol. Catal. A 2000, 160, 249–261. [Google Scholar] [CrossRef]
- Tafesse, T. Terapeutic agents useful for treating pain. PCT Int. Appl. WO2008/133973, 2008. [Google Scholar]
- Gulyás, H.; Rivilla, I.; Curreli, S.; Freixa, Z.; van Leeuwen, P.W.N.M. Highly active, chemo- and enantioselective Pt-SPO catalytic systems for the synthesis of aromatic carboxamides. Catal. Sci. Technol. 2015, 5, 3822–3828. [Google Scholar] [CrossRef]
- Jiang, X.-B.; Minnaard, A.J.; Feringa, B.L.; de Vries, J.G. Platinum-catalyzed selective hydration of hindered nitriles and nitriles with acid- and base-sensitive groups. J. Org. Chem. 2004, 69, 2327–2331. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Sato, S.; Yoshida, S.; Takada, K.; Itoh, M.; Seto, H.; Otake, N. Capuramycin, a new nucleoside antibiotic: Taxonomy, fermentation, isolation and characterization. J. Antibiot. 1986, 39, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Fukuoka, T.; Doi, N.; Harasaki, T.; Inoue, H.; Hotoda, H.; Kakuta, M.; Muramatsu, Y.; Yamamura, N.; Hoshi, M.; Hirota, T. Activity of capuramycin analogues against Mycobacterium tuberculosis, Mycobacterium avium and Mycobacterium intracellulare in vitro and in vivo. J. Antimicro. Chemother. 2004, 54, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Siricilla, S.; Aleiwi, B.A.; Kurosu, M. Improved synthesis of capuramycin and its analogues. Chem. Eur. J. 2013, 19, 13847–13858. [Google Scholar] [CrossRef] [PubMed]
- Kurosu, M.; Li, K.; Crick, D.C. Concise synthesis of capuramycin. Org. Lett. 2009, 11, 2393–2396. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.S.; Zhu, Y.L.; Yu, C.M.; Zhou, Y.Z.; Han, Y.Y.; Wu, F.W.; Qi, B.F. The structures of huperzine A and B, two new alkaloids exhibiting marked anticholinesterase activity. Can. J. Chem. 1986, 64, 837–839. [Google Scholar] [CrossRef]
- Kozikowski, A.P.; Tückmantel, W. Chemistry, pharmacology, and clinical efficacy of the Chinese nootropic agent huperzine A. Acc. Chem. Res. 1999, 32, 641–650. [Google Scholar] [CrossRef]
- Tun, M.K.M.; Herzon, S.B. The pharmacology and therapeutic potential of (−)-huperzine A. J. Exp. Pharmacol. 2012, 4, 113–123. [Google Scholar]
- Sohel, S.M.A.; Opatz, T. Synthetic approaches towards huperzine A and B. Arkivoc 2014, 1, 92–108. [Google Scholar] [CrossRef]
- Tun, M.K.M.; Wüstmann, D.J.; Herzon, S.B. A robust and scalable synthesis of the potent neuroprotective agent (−)-huperzine A. Chem. Sci. 2011, 2, 2251–2253. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Bella, M.; Chen, D.Y.-K.; Huang, X.; Ling, T.; Snyder, S.A. Total synthesis of diazonamide A. Angew. Chem. Int. Ed. 2004, 41, 3495–3499. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Chen, D.Y.-K.; Huang, X.; Ling, T.; Bella, M.; Snyder, S.A. Chemistry and biology of diazonamide A: First total synthesis and confirmation of the true structure. J. Am. Chem. Soc. 2004, 126, 12888–12896. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.-K.; Sammons, M.F.; Sammakia, T. A concise formal synthesis of diazonamide A by the stereoselective construction of the C10 quaternary center. Angew. Chem. Int. Ed. 2010, 49, 2397–2400. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, K.; Moore, R.E.; Bonjouklian, R.; Deeter, J.B.; Patterson, G.M.L.; Shaffer, S.; Smith, C.D.; Smitka, T.A. Welwitindolinones, unusual alkaloids from the blue-green algae Hapalosiphon welwitschii and Westiella intricate. Relationship to fischerindoles and hapalindoles. J. Am. Chem. Soc. 1994, 116, 9935–9942. [Google Scholar] [CrossRef]
- Jimenez, J.I.; Huber, U.; Moore, R.E.; Patterson, G.M.L. Oxidized welwitindolinones from terrestrial Fischerella spp. J. Nat. Prod. 1999, 62, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, J.C. Chemistry of the welwitindolinones. Top. Heterocycl. Chem. 2007, 11, 63–101. [Google Scholar]
- Greshock, T-J.; Funk, R.L. An approach to the total synthesis of welwistatin. Org. Lett. 2006, 8, 2643–2645. [Google Scholar]
- Jones, R.A.; Krische, M.J. Asymmetric total synthesis of the iridoid β-glucoside (+)-geniposide vis phosphine organocatalysis. Org. Lett. 2009, 11, 1849–1851. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, A.L.; Cassels, R.; Ready, S.J.; Warr, S.R. SB-203207 and SB-203208, two novel isoleucyl tRNA synthetase inhibitors from a Streptomyces sp. I. Fermentation, isolation and properties. J. Antibiot. 2000, 53, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Houge-Frydrych, C.S.V.; Gilpin, M.L.; Skett, P.W.; Tyler, J.W.S.R. SB-203207 and SB-203208, two novel isoleucyl tRNA synthetase inhibitors from a Streptomyces sp. II. Structure determination. J. Antibiot. 2000, 53, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, Y.; Ikeuchi, K.; Kawamoto, Y.; Akao, Y.; Furuta, T.; Asakawa, T.; Inai, M.; Wakimoto, T.; Fukuyama, T.; Kan, T. Enantioselective synthesis of SB-203207. Org. Lett. 2014, 16, 1646–1649. [Google Scholar] [CrossRef] [PubMed]
- Banwell, M.G.; Castro, C.F.; Easton, C.J.; Forrest, A.K.; Karoli, T.; March, D.R.; Mensah, L.; Nairn, M.R.; O´Hanlon, P.J.; Oldham, M.D.; Yue, W. Analogues of SB-203207 as inhibitors of tRNA synthetases. Bioorg. Med. Chem. Lett. 2000, 10, 2263–2266. [Google Scholar] [CrossRef]
- Kan, T.; Kawamoto, Y.; Asakawa, T.; Furuta, T.; Fukuyama, T. Synthetic studies on altemicidin: Stereocontrolled construction of the core framework. Org. Lett. 2008, 10, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Nising, C.F. Recent synthetic approaches towards the antiproliferative natural products avrainvillamide and stephacidin B. Chem. Soc. Rev. 2010, 39, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Herzon, S.B.; Myers, A.G. Enantioselective synthesis of stephacidin B. J. Am. Chem. Soc. 2005, 127, 5342–5344. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.G.; Herzon, S.B.; Wulff, J.E.; Siegrist, R.; Svenda, J.; Zajac, M.A. Synthesis of avrainvillamide, stephacidin B, and analogues thereof. PCT Int. Appl. WO2006/102097, 2006. [Google Scholar]
- Ohfune, Y.; Oe, K.; Namba, K.; Shinada, T. Total synthesis of manzacidins. An overview and perspective. Heterocycles 2012, 85, 2617–2649. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Deng, L. Dual-function cinchona alkaloid catalysis: Catalytic Asymmetric tandem conjugate addition-protonation for the direct creation of nonadjacent sterocenters. J. Am. Chem. Soc. 2006, 128, 3928–3930. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, H.; Wang, Y.; Wu, F. Asymmetric carbon-carbon-bond-forming reactions catalyzed by bifunctional cinchona alkaloids. US Pat. Appl. US2007/0083049, 2007. [Google Scholar]
- Wang, B.; Wu, F.; Wang, Y.; Liu, X.; Deng, L. Control of diasteroselectivity in tandem asymmetric reactions generating nonadjacent stereocenters with bifunctional catalysis by cinchona alkaloids. J. Am. Chem. Soc. 2007, 129, 768–769. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Cortez, F.; Sarpong, R. Ga(III)-catalyzed cycloisomerization approach to (±)-icetexone and (±)-epi-icetexone. Org. Lett. 2010, 12, 1428–1431. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Cortez, F.; Lapointe, D.; Hamlin, A.M.; Simmons, E.M.; Sarpong, R. Synthetic studies on the icetexones: Enantioselective formal synthesis of icetexone and epi-icetexone. Tetrahedron 2013, 69, 5665–5676. [Google Scholar] [CrossRef] [PubMed]
- Bielitza, M.; Pietruszka, J. The psymberin story―Biological properties and approaches towards total and analogue syntheses. Angew. Chem. Int. Ed. 2013, 52, 10960–10985. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; García-Fortanet, J.; De Brabander, J.K. Synthesis and complete stereochemical assignment of psymberin/irciniastatin A. J. Am. Chem. Soc. 2005, 127, 11254–11255. [Google Scholar] [CrossRef] [PubMed]
- De Brabander, J.K.; Jiang, X. Synthesis and complete stereochemical assignment of psymberin/irciniastatin for use as antitumor compounds. US Pat. Appl. US2007/0015821, 2007. [Google Scholar]
- Jiang, X.; Williams, N.; De Brabander, J.K. Synthesis of psymberin analogues: Probing a functional correlation with the pederin/mycalamide family of natural products. Org. Lett. 2007, 9, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Jiang, X.; De Brabander, J.K. Studies toward the unique pederin famility member psymberin: Full structure elucidation, two alternative total syntheses, and analogs. J. Am. Chem. Soc. 2012, 134, 17083–17093. [Google Scholar] [CrossRef] [PubMed]
- Rech, J.C.; Floreancig, P.E. Concise and stereoselective synthesis of the N7-C25 fragment of psymberin. Org. Lett. 2005, 7, 5175–5178. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.E.; Landaverry, Y.R.; Davies, J.R.; Milinkevich, K.A.; Ast, S.; Carlson, J.S.; Oliver, A.G.; Konopelski, J.P. Progress toward the total synthesis of psymberin/irciniastatin A. J. Org. Chem. 2009, 74, 5405–5410. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Marin, E.V.; García-Reynaga, P.; Romminger, S.; Pimenta, E.F.; Romeny, D.K.; Lodewyk, M.W.; Williams, D.E.; Andersen, R.J.; Miller, S.J.; Tantillo, D.J.; Berlinck, R.G.S.; Sarpong, R. Total synthesis and isolation of citrinalin and cyclopiamine congeners. Nature 2014, 509, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Marin, E.V.; Sarpong, R. Unified approach to prenylated indole alkaloids: Total synthesis of (−)-17-hydroxy-citrinalin B, (+)-stephacidin A, and (+)-notoamide I. Chem. Sci. 2015, 6, 5048–5052. [Google Scholar] [CrossRef]
- Yao, L.; Pitta, B.; Ravikumar, P.C.; Purzycki, M.; Fleming, F.F. Transmissive olefination route to putative “morinol I” lignans. J. Org. Chem. 2012, 77, 3651–3657. [Google Scholar] [CrossRef] [PubMed]
- Ripley, T.L.; Saseen, J.J. β-Blockers: A review of their pharmacological and physiological diversity in hypertension. Ann. Pharmacother. 2014, 48, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Akisanya, J.; Parkins, A.W.; Steed, J.W. A synthesis of atenolol using a nitrile hydration catalyst. Org. Process Res. Dev. 1998, 2, 274–276. [Google Scholar] [CrossRef]
- Craft, J. Eplerenone (Inspra), a new aldosterone antagonist for the treatment of systemic hypertension and heart failure. BUMC Proceedings 2004, 17, 217–220. [Google Scholar] [PubMed]
- Danjuma, M.I.; Mukherjee, I.; Makaronidis, J.; Osula, S. Converging indications of aldosterone antagonists (spironolactone and eplerenone): A narrative review of safety profiles. Curr. Hypertens. Rep. 2014, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gieling, R.G.; Groen, M.B. Process for making steroidal compounds. PCT Int. Appl. WO2006/097342, 2006. [Google Scholar]
- Metcalf, M.D.; Coop, A. Kappa opioid antagonists: Past, successes and future prospects. AAPS J. 2005, 7, E704–E722. [Google Scholar] [CrossRef] [PubMed]
- Carroll, F.I.; Carlezon, W.A., Jr. Development of κ opioid receptor antagonists. J. Med. Chem. 2013, 56, 2178–2195. [Google Scholar] [CrossRef] [PubMed]
- Urbano, M.; Guerrero, M.; Rosen, H.; Roberts, E. Antagonists of the kappa opioid receptor. Bioorg. Med. Chem. Lett. 2014, 24, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Brugel, T.A.; Smith, R.W.; Balestra, M.; Becker, C.; Daniels, T.; Hoerter, T.N.; Koether, G.M.; Throner, S.R.; Panko, L.M.; Folmer, J.J.; et al. Discovery of 8-azabicyclo[3.2.1]octan-3-yloxy-benzamides as selective antagonists of the kappa opioid receptor. Bioorg. Med. Chem. Lett. 2010, 20, 5847–5862. [Google Scholar] [CrossRef] [PubMed]
- Brugel, T.A.; Smith, R.W.; Balestra, M.; Becker, C.; Daniels, T.; Koether, G.M.; Throner, S.R.; Panko, L.M.; Brown, D.G.; Liu, R.; et al. SAR development of a series of 8-azabicyclo[3.2.1]octan-3-yloxy-benzamides as kappa opioid receptor antagonists. Bioorg. Med. Chem. Lett. 2010, 20, 5405–5410. [Google Scholar] [CrossRef] [PubMed]
- Saito, D.R.; Long, D.D.; Van Dyke, P.; Church, T.J.; Jiang, L.; Frieman, B. 8-Azabicyclo[3.2.1]octyl-2-hydroxybenzamide compounds as mu opioid receptor antagonists. PCT Int. Appl. WO2009/029256, 2009. [Google Scholar]
- Arnold, J.; Brugel, T.A.; Edwards, P.; Griffin, A.; Groblewski, T.; Labrecque, D.; Throner, S.; Wesolowski, S. Cyclopropyl amide derivatives ´978. PCT Int. Appl. WO2009/024823, 2009. [Google Scholar]
- Andrews, R.S.; Becker, J.J.; Gagné, M.R. A photoflow reactor for the continuous photoredox-mediated synthesis of C-glycoaminoacids and C-glycolipids. Angew. Chem. Int. Ed. 2012, 51, 4140–4143. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Tafesse, L. Nitrogen containing morphinan derivatives and the use thereof. PCT Int. Appl. WO2014/091298, 2014. [Google Scholar]
- Cobley, C.J.; van den Heuvel, M.; Abbadi, A.; de Vries, J.G. Platinum catalysed hydrolytic amidation of unactivated nitriles. Tetrahedron Lett. 2000, 41, 2467–2470. [Google Scholar] [CrossRef]
- Falck, J.R.; Gao, S.; Prasad, R.N.; Koduru, S.R. Electrophilic α-thiocyanation of chiral and achiral N-acyl amides. A convenient route to 5-substituted and 5,5-disubstituted 2,4-thiazolidinediones. Bioorg. Med. Chem. Lett. 2008, 18, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Finch, H.; Fox, C.; Sajad, M. Respiratory disease treatment. PCT Int. Appl. WO2010/015818, 2010. [Google Scholar]
- Li, J.; Chen, L.; Chin, E.; Lui, A.S.; Zecic, H. Platinum(II)-catalyzed intramolecular cyclization of alkynylbenzontriles: Synthesis of 1-alkoxyisoquinolines and isoquinolones. Tetrahedron Lett. 2010, 51, 6422–6425. [Google Scholar] [CrossRef]
- Brameld, K.A.; Carter, D.S.; Chin, E.; De Vicente Fidalgo, J.; Li, J.; Schoenfeld, R.C.; Sjogren, E.B.; Talamas, F.X. Heterocyclic antiviral compounds. PCT Int. Appl. WO2010/010017, 2010. [Google Scholar]
- Ahmed, T.J.; Fox, B.R.; Knapp, S.M.M.; Yelle, R.B.; Juliette, J.J.; Tyler, D.R. Investigation of the reactivity of Pt phosphinito and molybdocene nitrile hydration catalysts with cyanohydrins. Inorg. Chem. 2009, 48, 7828–7837. [Google Scholar] [CrossRef] [PubMed]
- Knapp, S.M.M.; Sherbow, T.J.; Ahmed, T.J.; Thiel, I.; Zakharov, L.N.; Juliette, J.J.; Tyler, D.R. Platinum phosphinito catalysts for nitrile hydration. J. Inorg. Organomet. Polym. 2014, 24, 145–156. [Google Scholar] [CrossRef]
- Knapp, S.M.M.; Sherbow, T.J.; Juliette, J.J.; Tyler, D.R. Cyanohydrin hydration with [Ru(η6-p-cymene)Cl2PR3] complexes. Organometallics 2012, 31, 2941–2944. [Google Scholar] [CrossRef]
- Knapp, S.M.M.; Sherbow, T.J.; Yelle, R.B.; Zakharov, L.N.; Juliette, J.J.; Tyler, D.R. Mechanistic investigations and secondary coordination sphere effects in the hydration of nitriles with [Ru(η6-arene)Cl2PR3] complexes. Organometallics 2013, 32, 824–834. [Google Scholar] [CrossRef]
- Knapp, S.M.M.; Sherbow, T.J.; Yelle, R.B.; Juliette, J.J.; Tyler, D.R. Catalytic nitrile hydration with [Ru(η6-p-cymene)Cl2(PR2R´)] complexes: Secondary coordination sphere effects with phosphine oxide and phosphinite ligands. Organometallics 2013, 32, 3744–3752. [Google Scholar] [CrossRef]
- Tomás-Mendivil, E.; Suárez, F.J.; Díez, J.; Cadierno, V. An efficient ruthenium(IV) catalyst for the selective hydration of nitriles to amides in water under mild conditions. Chem. Commun. 2014, 50, 9661–9664. [Google Scholar] [CrossRef] [PubMed]
- Sherbow, T.J.; Downs, E.L.; Sayler, R.I.; Razink, J.J.; Juliette, J.J.; Tyler, D.R. Investigation of 1,3,5-triaza-7-phosphaadamantane-stabilized silver nanoparticles as catalysts for the hydration of benzonitriles and acetone cyanohydrin. ACS Catal. 2014, 4, 3096–3104. [Google Scholar] [CrossRef]
- Downs, E.L.; Tyler, D.R. Nitrile and cyanohydrins hydration with nanoparticles formed in situ from a platinum dihydride complex. J. Inorg. Organomet. Polym. 2015, 25, 73–80. [Google Scholar] [CrossRef]
- Papakyprianou, A.; Parkins, A.W.; Prince, P.D.; Steed, J.W. Synthesis of O-protected mandelamide and its elaboration to 5-hydroxy-1,5-diphenylhydantoin. Org. Prep. Proced. Int. 2002, 34, 436–440. [Google Scholar] [CrossRef]
- North, M.; Parkins, A.W.; Shariff, A.N. Catalytic asymmetric synthesis of α-acetoxy amides. Tetrahedron Lett. 2004, 45, 7625–7627. [Google Scholar] [CrossRef]
- Trost, B.M.; Xie, J.; Sieber, J.D. The palladium catalyzed asymmetric addition of oxindoles and allenes: An atom-economical versatile method for the construction of chiral indole alkaloids. J. Am. Chem. Soc. 2011, 133, 20611–20622. [Google Scholar] [CrossRef] [PubMed]
- Grigg, R.; Hasakunpaisarn, A.; Kilner, C.; Kongkathip, B.; Kongkathip, N.; Pettman, A.; Sridharan, V. Catalytic processes for the functionalisation and desymmetrisation of malononitrile derivatives. Tetrahedron 2005, 61, 9356–9367. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadierno, V. Synthetic Applications of the Parkins Nitrile Hydration Catalyst [PtH{(PMe2O)2H}(PMe2OH)]: A Review. Appl. Sci. 2015, 5, 380-401. https://doi.org/10.3390/app5030380
Cadierno V. Synthetic Applications of the Parkins Nitrile Hydration Catalyst [PtH{(PMe2O)2H}(PMe2OH)]: A Review. Applied Sciences. 2015; 5(3):380-401. https://doi.org/10.3390/app5030380
Chicago/Turabian StyleCadierno, Victorio. 2015. "Synthetic Applications of the Parkins Nitrile Hydration Catalyst [PtH{(PMe2O)2H}(PMe2OH)]: A Review" Applied Sciences 5, no. 3: 380-401. https://doi.org/10.3390/app5030380
APA StyleCadierno, V. (2015). Synthetic Applications of the Parkins Nitrile Hydration Catalyst [PtH{(PMe2O)2H}(PMe2OH)]: A Review. Applied Sciences, 5(3), 380-401. https://doi.org/10.3390/app5030380