1. Introduction
White light-emitting-diodes (LEDs) have more advantages, such as energy saving, long lifetime, environmental friendly and high efficiency, compared with fluorescent lamps [
1,
2,
3]. At present, white LEDs can be obtained by combining near UV LED chips with blue/green/red tricolor phosphors, in order to obtain a higher efficiency white LED with appropriate color temperature and higher color-rendering index. This type of white LED is expected to dominate the market in the near future [
4,
5]. So it is important to find new tricolor phosphors for near UV LED chips.
The phosphors for near UV LED chips should exhibit intense broad emission with broad excitation band in near UV range. Compared with the phosphors activated by some trivalent rare earth ions (such as Eu
3+), Eu
2+ ions in many phosphors have broader emission with broad excitation band in near UV range [
6,
7,
8,
9], which are assigned to the 4f
65d
1 → 4f
7 transitions. In order to strengthen and broaden the absorption of phosphors in near UV range, one important approach is to introduce sensitizer to strengthen the excitation band in the phosphor. We consider that Bi
3+ is probably an eligible co-activator. On one hand, the introduction of Bi
3+ will influence their sub-lattice structure around the luminescent center ion. On the other hand, Bi
3+ is a very good sensitizer of luminescence in many hosts [
10,
11,
12]. It can efficiently absorb the UV-light and transfer the energy to the luminescent center, then the excitation band would be broadened and the emission intensity of the luminescent center would be strengthened.
Alkaline earth halo-silicates are well known good hosts for inorganic luminescent materials, due to their low synthesis temperature and high luminescence efficiency [
9]. Alkaline earth chlorosilicate Sr
4Si
3O
8Cl
4 can be served as the host of phosphors, which has an orthorhombic crystal structure [
13]. The luminescent properties of Sr
4Si
3O
8Cl
4 doped with Eu
2+ were firstly reported by Burrus
et al. [
13]. The luminescent properties of Sr
4Si
3O
8Cl
4 doped with Eu
2+ were optimized by co-doping with some divalent metal ions (such as Ca
2+, Mg
2+, Zn
2+) [
14,
15,
16].
In this paper, Sr4Si3O8Cl4 co-doped with Eu2+, Bi3+ were prepared by the high temperature reaction in the reduction atmosphere. The luminescent properties of Sr4Si3O8Cl4:Eu2+, Bi3+ were investigated. Finally, single blue-green LED was fabricated by combining the blue-green phosphor with ~395 nm emitting LED chips.
2. Results and Discussion
The XRD patterns of Sr
3.90Si
3O
8Cl
4:0.10Eu
2+ and Sr
3.50Si
3O
8Cl
4:0.10Eu
2+, 0.40Bi
3+ are shown in
Figure 1. They are almost consistent with the JCPDS card 40-0074 [Sr
4Si
3O
8Cl
4]. This indicates that the phosphor Sr
4Si
3O
8Cl
4:Eu
2+ almost shares the same phase as Sr
4Si
3O
8Cl
4, except for a few peaks of SrSiO
3 (marked by star shape). This result is in accordance with the references [
15,
16]. The impurity phase may be due to loss of chlorine content during the firing process. Eu
2+ and Bi
3+ maybe occupy the site of Sr
2+ in an octahedral site, because the ion radii of Bi
3+ (103 pm) and Eu
2+ (117 pm) are close to that of Sr
2+ (118 pm). Meanwhile, with the doping of Bi
3+/Eu
2+, a slight shift in the diffraction peaks toward higher angles can be found in
Figure 1. This shift is due to the difference of ionic radius between Bi
3+/Eu
2+ and Sr
2+.
Figure 2a is the excitation spectra of the phosphors Sr
4−4xSi
3O
8Cl
4:4
xEu
2+ (
x = 0.015, 0.020, 0.025, 0.030) by monitoring emission at 490 nm. These four curves are of similar shapes. The broad excitation band from 230 nm to 430 nm is due to the 4f–5d transitions of Eu
2+. These broad and intense excitation bands in near UV range match well with near-UV LED chip. When the content of Eu
2+ is 0.10, the excitation intensity is the strongest.
The emission spectra of Sr
4−4xSi
3O
8Cl
4:4
xEu
2+ (
x = 0.015, 0.020, 0.025, 0.030) under 320 nm excitation are shown in
Figure 2b. The blue-green emission band is due to the 4f
65d–4f
7 transition of Eu
2+. With the increase of the Eu
2+ content, the intensity of the blue-green emission becomes higher. When the content of Eu
2+ is 0.10, the emission intensity is the strongest.
Figure 1.
X-ray powder diffraction (XRD) patterns of Sr3.90Si3O8Cl4:0.10Eu2+ and Sr3.50Si3O8Cl4:0.10Eu2+, 0.40Bi3+. The Asterisk represents the peak of SrSiO3.
Figure 1.
X-ray powder diffraction (XRD) patterns of Sr3.90Si3O8Cl4:0.10Eu2+ and Sr3.50Si3O8Cl4:0.10Eu2+, 0.40Bi3+. The Asterisk represents the peak of SrSiO3.
Figure 2.
(a) Excitation spectra and (b) emission spectra of Sr4–4xSi3O8Cl4:4xEu2+ (x = 0.015, 0.020, 0.025, 0.030).
Figure 2.
(a) Excitation spectra and (b) emission spectra of Sr4–4xSi3O8Cl4:4xEu2+ (x = 0.015, 0.020, 0.025, 0.030).
Since the luminescent mechanism of Eu
2+ is the 4f–5d allowed electric-dipole transition, the energy transfer process of Eu
2+ in the Sr
4Si
3O
8Cl
4 phosphors would be attributed to an electric multipole-mutipole interaction. When the energy transfer occurs between the same sites of activators, the intensity of multipole interaction can be determined by the change of the emission intensity from the emitting level. According to the report of Van Uitert [
17], the emission intensity (
I) of per activator ion follows the equation [
18,
19]:
where
I is the emission intensity, χ is the Eu
2+ concentration,
K and β are constants for the same excitation condition for a given host crystal. Q is a constant of multipole interaction equals to 3, 6, 8 or 10 for energy transfer among the nearest-neighbor ions, dipole-dipole (d–d), dipole-quadruple (d–q) or quadruple-quadruple (q–q) interaction, respectively. To get a Q value for the emission center, the plot of log (
I/χ) as a function of log (χ) for the Sr
4−4xSi
3O
8Cl
4:4
xEu
2+ phosphors is plotted, as shown in
Figure 3. It can be seen that a linear relation between log (
I/χ) and log (χ) is found and the slope is −0.5299. The Q value can be obtained as 1.5897. That means the quenching is directly proportional to the activator ion concentration, which indicates that the concentration quenching is caused by the energy transfer among the nearest-neighbor Eu
2+ ions in the Sr
4−4xSi
3O
8Cl
4: 4
xEu
2+ phosphors.
Figure 3.
The plot of log (I/x) as a function of log (x) for the Sr4−4xSi3O8Cl4:4xEu2+phosphors (λex = 320 nm).
Figure 3.
The plot of log (I/x) as a function of log (x) for the Sr4−4xSi3O8Cl4:4xEu2+phosphors (λex = 320 nm).
The excitation spectra of the phosphors Sr
3.90−4ySi
3O
8Cl
4:0.10Eu
2+, 4
yBi
3+ (
y = 0.00, 0.05, 0.10, 0.15, 0.20) by monitoring emission 490 nm is shown in
Figure 4. With the doping of Bi
3+, the shoulder peak around 400 nm is broadened with a little red shift, and the excitation intensity is enhanced. When the content of Bi
3+ is 0.10, the excitation intensity is the strongest.
Figure 4.
Excitation spectra of Sr3.90−4ySi3O8Cl4:0.10Eu2+, 4yBi3+ (y = 0.00, 0.05, 0.10, 0.15, 0.20) by monitoring emission at 490 nm.
Figure 4.
Excitation spectra of Sr3.90−4ySi3O8Cl4:0.10Eu2+, 4yBi3+ (y = 0.00, 0.05, 0.10, 0.15, 0.20) by monitoring emission at 490 nm.
Figure 5 is the emission spectra of Sr
3.90−4ySi
3O
8Cl
4:0.10Eu
2+, 4
yBi
3+ (
y = 0.00, 0.05, 0.10, 0.15, 0.20) under 320 nm excitation. The broad emission from 400 nm to 600 nm is due to the 4f
65d
1 → 4f
7 transition of Eu
2+ ions. The strongest peak is located at 490 nm. The concentration dependence of the relative integral emission intensity of Sr
3.90−4ySi
3O
8Cl
4:0.10Eu
2+, 4
yBi
3+ is shown in the inserted figure of
Figure 5. When the content of Bi
3+ is at 0.10, its emission intensity is the strongest, and its intensity is about 1.3 times higher than that of Sr
3.90Si
3O
8Cl
4:0.10Eu
2+ without Bi
3+. This result is consistent with their excitation spectra. Bright blue-green light can be observed from Sr
3.50Si
3O
8Cl
4:0.10Eu
2+, 0.40Bi
3+ under 365 nm light excitation (seeing the inset of
Figure 5). The compositions Sr
3.90−4ySi
3O
8Cl
4:0.10Eu
2+, 4
yBi
3+ are not charge balanced and have a slight excess of positive charge because of Bi
3+ substituting Sr
2+. The excess charge could be compensated by a number of mechanisms, for example, cation vacancies, varying slightly O contents,
etc. [
20]. In this series of phosphors, this kind of substitution did not influence the emission spectra shapes of Sr
3.90−4ySi
3O
8Cl
4:0.10Eu
2+, 4
yBi
3+ (seeing
Figure 5).
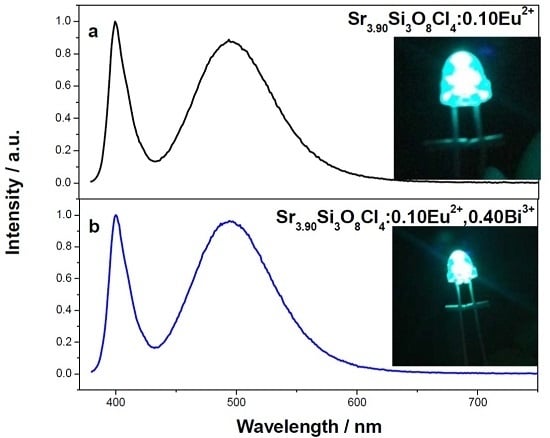
Figure 6 shows the electroluminescence (EL) spectra of the intense blue-green LED fabricated with Sr
3.90Si
3O
8Cl
4:0.10Eu
2+ and Sr
3.50Si
3O
8Cl
4:0.10Eu
2+, 0.40Bi
3+ under 20 mA current excitation. The emission band at ~400 nm is attributed to the emission of LED chip and the broad band from 430 nm to 600 nm is due to the emission of the phosphor. Comparing with curve
a, the ratio of LED chip emission to phosphor emission in curve
b is smaller. The result indicates phosphor Sr
3.50Si
3O
8Cl
4:0.10Eu
2+, 0.40Bi
3+ can more efficiently absorb the emission of LED chip, and exhibit stronger blue-green emission, compared with Sr
3.90Si
3O
8Cl
4:0.10Eu
2+. Bright blue-green light is observed from these two LEDs by the naked eye. This result is in accordance with their emission spectra. Their CIE chromaticity coordinates are calculated to be
x = 0.169,
y = 0.356, and
x = 0.170,
y = 0.362, respectively.
Figure 5.
Emission spectra of Sr3.90−4ySi3O8Cl4:0.10Eu2+, 4yBi3+ (y = 0.00, 0.05, 0.10, 0.15, 0.20) under 320 nm excitation, the inserted figures are the concentration dependence of the relative integral emission intensity of Sr3.90−4ySi3O8Cl4:0.10Eu2+, 4yBi3+ and the image of Sr3.50Si3O8Cl4:0.10Eu2+, 0.40Bi3+ under 365 nm excitation.
Figure 5.
Emission spectra of Sr3.90−4ySi3O8Cl4:0.10Eu2+, 4yBi3+ (y = 0.00, 0.05, 0.10, 0.15, 0.20) under 320 nm excitation, the inserted figures are the concentration dependence of the relative integral emission intensity of Sr3.90−4ySi3O8Cl4:0.10Eu2+, 4yBi3+ and the image of Sr3.50Si3O8Cl4:0.10Eu2+, 0.40Bi3+ under 365 nm excitation.
Figure 6.
Electroluminescence (EL) spectra of the blue-green light-emitting-diodes (LEDs) based on (a) Sr3.90Si3O8Cl4:0.10Eu2+ and (b) Sr3.50Si3O8Cl4:0.10Eu2+, 0.40Bi3+ under 20 mA current excitation; the inserted figures are the images of these two LEDs.
Figure 6.
Electroluminescence (EL) spectra of the blue-green light-emitting-diodes (LEDs) based on (a) Sr3.90Si3O8Cl4:0.10Eu2+ and (b) Sr3.50Si3O8Cl4:0.10Eu2+, 0.40Bi3+ under 20 mA current excitation; the inserted figures are the images of these two LEDs.
3. Experimental Section
The phosphors Sr4Si3O8Cl4 doped with Eu2+ and Bi3+ were synthesized by a solid-state reaction. The raw materials SrCO3 (A.R. grade, Aladdin, Shanghai, China), SiO2 (A.R. grade, Aladdin, Shanghai, China), SrCl2·6H2O (A.R. grade, Aladdin, Shanghai, China), Bi2O3 (A.R. grade, Aladdin, Shanghai, China) and Eu2O3 (99.99% purity, Aladdin, Shanghai, China) were thoroughly mixed and ground together according to the given stoichiometric ratio. The mixture was first pre-fired at 400 °C for 2 h, and heated at 950 °C for 4 h. The process reduced CO atmosphere. The blue-green LED was fabricated by combing an LED chip (~395 nm) with the mixture of blue-green phosphor and epoxy resin (the ratio of mass is 1:1).
The structure of these phosphors was recorded by X-ray powder diffraction (XRD) using Cu Kα radiation on a RIGAKU D/max 2200 vpc X-ray Diffractometer (Rigaku Corporation, Osaka, Japan). Their photoluminescent spectra were recorded on a Cary Eclipse FL1011M003 (Varian Palo Alto, CA, USA) spectrofluorometer and the xenon lamp was used as excitation source. The electro-luminescence of blue-green LEDs was recorded on a high-accuracy array spectrometer (HSP6000, HongPu Optoelectronics Technology Co. Ltd., Hangzhou, China). All the measurements were performed at room temperature.