Main Leaf Polyphenolic Components of Berry Color Variant Grapevines and Their Acclimative Responses to Sunlight Exposure
Abstract
:1. Introduction
2. Experimental Section
2.1. Plant Material, Light Conditions
2.2. Reagents
2.3. Sample Preparation
2.4. Chromatographic Separation
2.5. HPLC-ESI-qTOFMS Analysis
2.6. Confocal Laser Scanning Microscopy (CLSM) and Flavonoid Fluorescence Detection
2.7. Statistics
3. Results and Discussion
3.1. Identification of Major Polyphenols
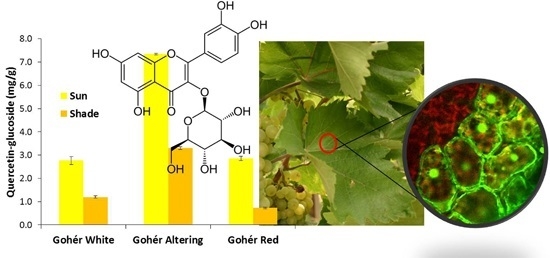
3.2. Changes in Leaf Polyphenolic Composition
| Compounds | Leaf Polyphenolic Profile [mg g−1] Dry Weight | |||||
|---|---|---|---|---|---|---|
| Gohér White | Gohér Altering | Gohér Red | ||||
| Sun | Shade | Sun | Shade | Sun | Shade | |
| Phenolic acid | ||||||
| trans-caftaric acid | 4.00 ± 0.075 | *3.73 ± 0.046 | 8.46 ± 0.314 | *6.20 ± 0.245 | 3.66 ± 0.071 | *1.94 ± 0.075 |
| Flavonol-glycosides | ||||||
| Quercetin-glucuronide | 8.87 ± 0.377 | 8.02 ± 0.394 | 18.52 ± 0.386 | *14.42 ± 0.173 | 8.42 ± 0.177 | *6.19 ± 0.102 |
| Quercetin-glucoside | 2.77 ± 0.168 | *1.20 ± 0.054 | 7.35 ± 0.036 | *3.30 ± 0.059 | 2.86 ± 0.091 | *0.73 ± 0.030 |
| Quercetin-rutinoside | 0.64 ± 0.044 | *0.43 ± 0.031 | 1.65 ± 0.018 | *1.06 ± 0.033 | 0.50 ± 0.020 | *0.13 ± 0.004 |
| Quercetin-galactoside | 0.59 ± 0.042 | *0.26 ± 0.017 | 1.81 ± 0.036 | *0.73 ± 0.020 | 0.57 ± 0.010 | *0.13 ± 0.005 |
| Kaempferol-glucuronide | 0.56 ± 0.025 | 0.56 ± 0.027 | 1.69 ± 0.048 | *1.31 ± 0.024 | 0.43 ± 0.006 | *0.24 ± 0.007 |
| Kaempferol-glucoside | 0.53 ± 0.031 | *0.21 ± 0.011 | 1.76 ± 0.027 | *0.54 ± 0.005 | 0.46 ± 0.013 | *0.11 ± 0.004 |
| Kaempferol-rutinoside | 0.18 ± 0.008 | 0.17 ± 0.010 | 0.73 ± 0.010 | *0.42 ± 0.011 | 0.13 ± 0.006 | *0.07 ± 0.003 |
| Total amount | 18.14 ± 0.771 | 14.58 ± 0.590 | 41.97 ± 0.876 | 27.98 ± 0.571 | 17.02 ± 0.393 | 9.54 ± 0.229 |
| Sun Leaves | G. White | G. Altering | ||
| G. Altering | 39.13 | |||
| G. Red | 2.52 | 58.32 | ||
| Shade Leaves | G. White | G. Altering | ||
| G. Altering | −34.86 | |||
| G. Red | 13.98 | 85.45 | ||
| Shade Leaves | Sun Leaves | |||
| G. White | G. Altering | G. Red | ||
| G. White | 6.41 | 50.62 | 7.01 | |
| G. Altering | −20.70 | 30.56 | −56.21 | |
| G. Red | 18.86 | 74.09 | 52.83 | |
3.3. Changes in Leaf Flavonoid Distribution

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
| Retention Time, min | UV max | MS Polarity | Supposed Compound and Annotation of Diagnostic Ions | Elemental Composition of Diagnostic Ions (Ion Formula) | Expected m/z | Error, ppm |
|---|---|---|---|---|---|---|
| 6.32 | 325 | − | Caftaric acida | C13H11O9 | 311.0409 | −0.32 |
| Caffeic acid residue | C9H7O4 | 179.035 | 0.83 | |||
| Caffeic acid residue-CO2 | C8H7O2 | 135.0452 | −1.02 | |||
| Tartaric acid residue | C4H5O6 | 149.0092 | 0.31 | |||
| 6.94 | 325 | − | Caftaric acid isomer | C13H11O9 | 311.0409 | 2.77 |
| Caffeic acid residue | C9H7O4 | 179.035 | 4.29 | |||
| Caffeic acid residue-CO2 | C8H7O2 | 135.0452 | 5.53 | |||
| Tartaric acid residue | C4H5O6 | 149.0092 | 4.99 | |||
| [[2M − H]− | C26H23O18 | 623.089 | 0.33 | |||
| 10.91 | 315 | − | Coutaric acid | C13H11O8 | 295.0459 | 4.34 |
| Tartaric acid residue | C4H5O6 | 149.0092 | 6.85 | |||
| Coumaric acid residue | C9H7O3 | 163.0401 | 9.46 | |||
| 17.3 | 260, 360 | + | Myr − Hx 1 | |||
| Myr | C15H11O8 | 319.0448 | 0.91 | |||
| Myr + Hx | C21H21O13 | 481.0977 | 3.53 | |||
| Myr + Hx + Na | C21H20O13Na | 503.0796 | 3.68 | |||
| 17.47 | 265, 360 | + | Myr − Hx 2 | |||
| Myr | C15H11O8 | 319.0448 | −1.35 | |||
| Myr + Hx | C21H21O13 | 481.0977 | 2.23 | |||
| Myr + Hx + Na | C21H20O13Na | 503.0796 | 3.68 | |||
| 19.23 | 260, 360 | + | Que − 3-O-rutinosidea | |||
| Que | C15H11O7 | 303.05 | −3.1 | |||
| Que + dHx | C21H21O11 | 449.1079 | −1.02 | |||
| Que + Hx | C21H21O12 | 465.1028 | −2.69 | |||
| Que + dHx + Hx | C27H31O16 | 611.1607 | −0.93 | |||
| Que + dHx + Hx + Na | C27H30O16Na | 633.1432 | −1.43 | |||
| 19.68 | 260, 360 | + | Que − 3-O-galactosidea | |||
| Que | C15H11O7 | 303.05 | 4.00 | |||
| Que + Hx | C21H21O12 | 465.1028 | 5.15 | |||
| Que + Hx + Na | C21H20O12Na | 487.0853 | 5.66 | |||
| 19.81 | 255, 360 | + | Que − 3-O-glucuronidea | |||
| Que | C15H11O7 | 303.05 | −5.66 | |||
| Que + Hxa | C21H19O13 | 479.082 | 4.19 | |||
| Que + Hxa + Na | C21H18O13Na | 501.064 | −4.68 | |||
| 19.95 | 255, 360 | + | Que − 3-O-glucosidea | |||
| Que | C15H11O7 | 303.05 | −1.52 | |||
| Que + Hx | C21H21O12 | 465.1028 | −0.56 | |||
| Que + Hx + Na | C21H20O12Na | 487.0853 | 0.70 | |||
| 21.05 | 260, 350 | + | Kae − 3-O-rutinosidea | |||
| Kae | C15H11O6 | 287.055 | 5.64 | |||
| Kae + Hx | C21H21O11 | 449.1078 | 7.34 | |||
| Kae + Hx + dHx | C27H31O15 | 595.1657 | 8.05 | |||
| Kae + Hx + dHx + Na | C27H30O15Na | 617.1482 | 8.32 | |||
| 21.23 | 260, 350 | + | Kae − 3-O-glucosidea | |||
| Kae | C15H11O6 | 287.055 | 3.56 | |||
| Kae + Hx | C21H21O11 | 449.1078 | 4.17 | |||
| Kae + Hx + Na | C21H20O11Na | 471.0898 | 5.34 | |||
| 21.97 | 260, 350 | + | Kae − Hx 2 | |||
| Kae | C15H11O6 | 287.055 | 3.24 | |||
| Kae + Hx | C21H21O11 | 449.1078 | 4.83 | |||
| Kae + Hx + Na | C21H20O11Na | 471.0898 | 4.84 | |||
| 21.99 | 260, 350 | + | Kae − 3-O-glucuronidea | |||
| Kae | C15H11O6 | 287.055 | −3.32 | |||
| Kae + Hxa | C21H19O12 | 463.0871 | −2.22 | |||
| Kae + Hxa + Na | C21H18O12Na | 485.069 | 4.95 | |||
| 21.47 | 265, 350 | + | Isr + Hx + dHx | |||
| Isr | C16H13O7 | 317.0656 | 3.50 | |||
| Isr + Hx | C22H23O12 | 479.1184 | −2.70 | |||
| Isr + Hx + dHx | C28H33O16 | 625.1763 | 3.46 | |||
| Isr + Hx + dHx + Na | C28H32O16Na | 647.1583 | 5.30 | |||
| 22.12 | 265, 350 | + | Isr + Hx 1 | |||
| Isr | C16H13O7 | 317.0656 | 2.52 | |||
| Isr + Hx | C22H23O12 | 479.1184 | −5.85 | |||
| Isr + Hx + Na | C22H22O12Na | 501.1003 | −1.29 | |||
| 22.44 | 265, 350 | + | Isr + Hx 2 | |||
| Isr | C16H13O7 | 317.0656 | 1.31 | |||
| Isr + Hx | C22H23O12 | 479.1184 | 1.87 | |||
| Isr + Hx + Na | C22H22O12Na | 501.1003 | 3.84 |
| Sun / Shade Leaves | |||
|---|---|---|---|
| Polyphenolic Compounds | Gohér White | Gohér Altering | Gohér Red |
| trans-caftaric-acid | 5.31 | 9.83 | 28.75 |
| Quercetin-glucuronide | 2.69 | 16.79 | 18.88 |
| Quercetin-glucoside | 15.43 | 101.07 | 38.55 |
| Quercetin-rutinoside | 6.74 | 27.33 | 31.73 |
| Quercetin-galactoside | 12.41 | 44.96 | 68.00 |
| Kaempferol-glucuronide | −0.15 | 12.11 | 35.50 |
| Kaempferol-glucoside | 16.98 | 77.34 | 46.24 |
| Kaempferol-rutinoside | 2.21 | 35.33 | 15.19 |
References
- Berli, F.; D’Angelo, J.; Cavagnaro, B.; Bottini, R.; Wuilloud, R.; Silva, M.F. Phenolic composition in grape (Vitis vinifera L. cv. Malbec) ripened with different solar UV-B radiation levels by capillary zone electroforesis. J. Agric. Food Chem. 2008, 56, 2892–2898. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Oliboni, L.S.; Agostini, F.; Funchal, C.; Serafini, L.; Henriques, J.A.; Salvador, M. Phenolic content of grapevine leaves (Vitis labrusca var. Bordo) and its neuroprotective effect against peroxide damage. Toxicol. In Vitro 2010, 24, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Kosar, M.; Küpeli, E.; Malyer, H.; Uylaser, V.; Türkben, C.; Baser, K.H. Effect of brining on biological activity of leaves of Vitis vinifera L. (cv. Sultani Cekirdeksiz) from Turkey. J. Agric. Food Chem. 2007, 55, 4596–4603. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Hernández-Ledesma, B.; Gómez-Cordovés, C.; Bartolomé, B. Commercial dietary ingredients from Vitis vinifera L. leaves and grape skins: Antioxidant and chemical characterization. J. Agric. Food Chem. 2006, 54, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastiani, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 21, 589–598. [Google Scholar] [CrossRef]
- Leifert, W.R.; Abeywardena, M.Y. Cardioprotective actions of grape polyphenols. Nutr. Res. 2008, 28, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Markham, K.R. Flavones, Flavonols and their glycosides. Methods in Plant Biochemistry; Harborne, J.B., Ed.; Academic Press Ltd.: London, UK, 1989; Volume 1, pp. 197–235. [Google Scholar]
- Schoedl, K.; Schuhmacher, R.; Forneck, A. Studying the polyphenols of grapevine leaves according to age and insertion level under controlled conditions. Sci. Hortic. 2012, 141, 37–41. [Google Scholar] [CrossRef]
- Chacón, J.L.; García, E.; Martínez, J.; Romero, R.; Gómez, S. Impact of the vine water status on the berry and seed phenolic composition of ‘Merlot’ (Vitis vinifera L.) cultivated in a warm climate: Consequence for the style of wine. Vitis 2009, 48, 7–9. [Google Scholar]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Viticul. 2006, 57, 257–268. [Google Scholar]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011, 6, 1720–1731. [Google Scholar] [PubMed]
- Tattini, M.; Galardi, C.; Pinelli, P.; Massai, R.; Remorini, D.; Agati, G. Differential accumulation of flavonoids and hydroxycinnamates in leaves of Ligustrum vulgare under excess light and drought stress. New Phytol. 2004, 163, 547–561. [Google Scholar] [CrossRef]
- Tattini, M.; Remorini, D.; Pinelli, P.; Agati, G.; Saracini, E.; Traversi, M.L.; Massai, R. Morpho-anatomical, physiological and biochemical adjustments in response to root zone salinity stress and high solar radiation in two Mediterranean evergreen schrubs, Myrtus communis and Pistacia lentiscus. New Phytol. 2006, 170, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Majer, P.; Neugart, S.; Krumbein, A.; Schreiner, M.; Hideg, É. Singlet oxygen scavenging by leaf flavonoids contributes to sunlight acclimation in Tilia platyphyllos. Environ. Exp. Bot. 2014, 100, 1–9. [Google Scholar] [CrossRef]
- Di Ferdinando, M.; Brunetti, C.; Agati, G.; Tattini, M. Multiple functions of polyphenols in plants inhabiting unfavorable Mediterranean areas. Environ. Exp. Bot. 2014, 103, 107–116. [Google Scholar] [CrossRef]
- Rozema, J.; van de Staaij, J.; Björn, L.O.; Caldwell, M.M. UV-B as an environmental factor in plant life: Stress and regulation. Trends. Ecol. Evol. 1997, 12, 22–28. [Google Scholar] [CrossRef]
- Hutzler, P.; Fischbach, R.; Heller, W.; Jungblut, T.P.; Reuber, S.; Schmitz, R.; Veit, M.; Weissenbck, G.; Schnitzler, J.P. Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. Exp. Bot. 1998, 49, 953–965. [Google Scholar] [CrossRef]
- Kolb, C.A.; Kaser, M.A.; Kopecky, J.; Zotz, G.; Riederer, M.; Pfündel, E.E. Effects of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant Physiol. 2001, 127, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Götz, M.; Albert, A.; Stitch, S.; Heller, W.; Scherb, H.; Krins, A.; Langebartels, C.; Seidlitz, H.K.; Ernst, D. PAR modulation of the UV-dependent levels of flavonoid metabolites in Arabidopsis thaliana (L.) Heynh. leaf rosettes: Cumulative effects after a whole vegetative growth period. Protoplasma 2010, 243, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants and Developmental Regulators: Relative Significance in Plants and Humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [PubMed]
- Cortell, J.M.; Kennedy, J.A. Effect of shading on accumulation of flavonoid compounds in (Vitis vinifera L.) Pinot noir fruit and extraction in a model system. J. Agric. Food Chem. 2006, 55, 8510–8520. [Google Scholar] [CrossRef] [PubMed]
- Matus, J.T.; Loyola, R.; Vega, A.; Pena-Neira, A.; Bordeu, E.; Arce-Johnson, P.; Alcalde, J.A. Post-veraison sunlight exposure induces MYB-mediated transcriptional regulation of anthocyanin and flavonol synthesis in berry skins of Vitis vinifera. J. Exp. Bot. 2009, 60, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo-González, M.; Martínez-Carballo, E.; Cancho-Grande, B.; Santiago, J.L.; Martínez, M.C.; Simal-Gándara, J. Pattern recognition of three Vitis vinifera L. red grapes varieties based on anthocyanin and flavonol profiles, with correlations between their biosynthesis pathways. Food Chem. 2012, 13, 9–19. [Google Scholar] [CrossRef]
- Galbács, Z.; Molnár, S.; Halász, G.; Kozma, P.; Hoffmann, S.; Kovács, L.; Veres, A.; Galli, Z.; Szőke, A.; Heszky, L.; et al. Identification of grapevine cultivars using microsatellite-based DNA barcodes. Vitis 2009, 48, 17–24. [Google Scholar]
- Castellarin, S.D.; di Gaspero, G.; Marconi, R.; Nonis, A.; Peterlunger, E.; Paillard, S.; Adam-Blondon, A.F.; Testolin, R. Colour variation in red grapevines (Vitis vinifera L.): Genomic organisation, expression of flavonoid 3′-hydroxylase, flavonoid 3′,5′-hydroxylase genes and related metabolite profiling of red cyanidin-/blue delphinidin-based anthocyanins in berry skin. BMC Genomics 2006, 7. [Google Scholar] [CrossRef] [PubMed]
- Németh, M. Ampelographic Album: Cultivated Grapevine Varieties II, 1st ed.; Agricultural Press: Budapest, Hungary, 1970; pp. 69–70. [Google Scholar]
- Abrankó, L.; Garcia-Reyes, J.F.; Molina-Diaz, A. Systemic bottom-up approach for flavonoid derivative screening in plant material using liquid chromatography high-resolution mass spectrometry. Anal. Bioanal. Chem. 2012, 403, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Traldi, P. Grape and Wine Polyphenols. In Mass Spectrometry in Grape and Wine Chemistry; Desiderio, D.M., Nibbering, N.M.M., Eds.; Wiley & Sons Inc.: Hoboken, NJ, USA, 2010; Part II; pp. 163–214. [Google Scholar]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- Taware, P.B.; Dhumal, K.N.; Oulkar, D.P.; Patil, S.H.; Banerjee, K. Phenolic alterations in grape leaves, berries and wines due to foliar and cluster powdery mildew infections. Int. J. Pharm. Biol. Sci. 2010, 1, 1–14. [Google Scholar]
- Balik, J.; Kyselakova, M.; Vrchotova, N.; Triska, J.; Kumsta, M.; Veverka, J.; Hic, P.; Totusek, J.; Lefernova, D. Relations between polyphenols content an antioxidant activity in vine grapes and leaves. Chez. J. Food Sci. 2008, 26, 25–32. [Google Scholar]
- Doshi, P.; Adsule, P.; Banerjee, K. Phenolic composition and antioxidant activity in grapevine parts and berries (Vitis vinifera L.) cv. Kishmish Chornyi (Sharad Seedless) during maturation. Int. J. Food Sci. Technol. 2006, 41, 1–9. [Google Scholar] [CrossRef]
- Mullineaux, P.; Karpinski, S. Signal transduction in response to excess light: Getting out of the chloroplast. Curr. Opin. Plant Biol. 2002, 5, 43–48. [Google Scholar] [CrossRef]
- Hatier, J.H.B.; Gould, K.S. Foliar anthocyanins as modulators of stress signals. J. Theor. Biol. 2008, 253, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Polster, J.; Dithmar, H.; Burgemeister, R.; Friedemann, G.; Feucht, W. Flavonoids in plant nuclei: Detection by laser microdissection and pressure catapulting (LMPC), in vivo staining, and UV-visible spectroscopic titration. Physiol. Plantarum. 2006, 126, 163–174. [Google Scholar] [CrossRef]
- Pérez-Gregorio, M.R.; Regueiro, J.; González-Barreiro, C.; Rial-Otero, R.; Simal-Gándara, J. Changes in antioxidant flavonoids during freeze-drying of red onions and subsequent storage. Food Cont. 2011, 22, 1108–1113. [Google Scholar] [CrossRef]
- Inoue, K. Emerging roles of the chloroplast outer envelope membrane. Trends Plant Sci. 2011, 16, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Erlejman, A.G.; Verstraiten, S.V.; Graga, C.G.; Oteiza, P.I. The interaction of flavonoids with membranes: Potential determinants of flavonoid antioxidant effects. Free Radical Res. 2004, 38, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Stefano, G.; Biricolti, S.; Tattini, M. Mesophyll distribution of ‘antioxidant’ flavonoid glycosides in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann. Bot-London 2009, 104, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Biricolti, S.; Guidi, L.; Ferrini, F.; Fini, A.; Tattini, M. The biosynthesis of flavonoids is enhanced similarly by UV radiation and root zone salinity in L. vulgare leaves. J. Plant Phys. 2011, 168, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochem 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Tattini, M.; Guidi, L.; Morassi-Bonzi, L.; Pinelli, P.; Remorini, D.; Degl’Innocenti, E.; Giordano, C.; Massai, R.; Agati, G. On the role of flavonoids in the integrated mechanisms of response of Ligustrum vulgare and Phillyrea latifolia to high solar radiation. New Phytol 2005, 167, 457–470. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocsis, M.; Abrankó, L.; Ayaydin, F.; Csepregi, K.; Papp, N.; Teszlák, P.; Jakab, G. Main Leaf Polyphenolic Components of Berry Color Variant Grapevines and Their Acclimative Responses to Sunlight Exposure. Appl. Sci. 2015, 5, 1955-1969. https://doi.org/10.3390/app5041955
Kocsis M, Abrankó L, Ayaydin F, Csepregi K, Papp N, Teszlák P, Jakab G. Main Leaf Polyphenolic Components of Berry Color Variant Grapevines and Their Acclimative Responses to Sunlight Exposure. Applied Sciences. 2015; 5(4):1955-1969. https://doi.org/10.3390/app5041955
Chicago/Turabian StyleKocsis, Marianna, László Abrankó, Ferhan Ayaydin, Kristóf Csepregi, Nóra Papp, Péter Teszlák, and Gábor Jakab. 2015. "Main Leaf Polyphenolic Components of Berry Color Variant Grapevines and Their Acclimative Responses to Sunlight Exposure" Applied Sciences 5, no. 4: 1955-1969. https://doi.org/10.3390/app5041955
APA StyleKocsis, M., Abrankó, L., Ayaydin, F., Csepregi, K., Papp, N., Teszlák, P., & Jakab, G. (2015). Main Leaf Polyphenolic Components of Berry Color Variant Grapevines and Their Acclimative Responses to Sunlight Exposure. Applied Sciences, 5(4), 1955-1969. https://doi.org/10.3390/app5041955