Tunable Degradation Rate and Favorable Bioactivity of Porous Calcium Sulfate Scaffolds by Introducing Nano-Hydroxyapatite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Fabrication
2.2. Characterization
2.3. SBF Immersion
2.4. Cell Culture
2.5. Statistical Analysis
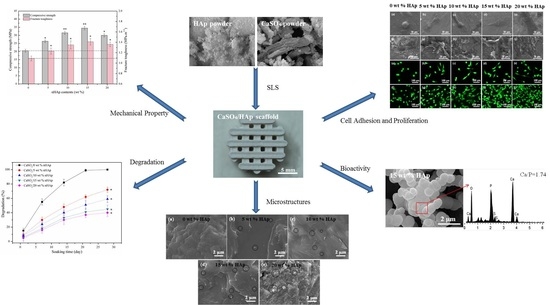
3. Results and Discussion
3.1. Scaffolds Fabrication
3.2. Phase and Microstructures
3.3. Degradability and pH
3.4. Mechanical Property
3.5. Bioactivity
3.6. Cell Adhesion and Proliferation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794. [Google Scholar]
- Martínez-Vázquez, F.J.; Cabanas, M.V.; Paris, J.L.; Lozano, D.; Vallet-Regí, M. Fabrication of novel Si-doped hydroxyapatite/gelatine scaffolds by rapid prototyping for drug delivery and bone regeneration. Acta Biomater. 2015, 15, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Chen, C.; Tsai, X.T. Visualizing the Knowledge Domain of Nanoparticle Drug Delivery Technologies: A Scientometric Review. Appl. Sci. 2016, 6, 11. [Google Scholar] [CrossRef]
- Thuaksuban, N.; Luntheng, T.; Monmaturapoj, N. Physical characteristics and biocompatibility of the polycaprolactone–biphasic calcium phosphate scaffolds fabricated using the modified melt stretching and multilayer deposition. J. Biomater. Appl. 2016, 30, 1460–1472. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Xu, D.P.; Liu, Z.M.; Du, Y.; Wang, X.K. Synthesis of the New-Type Vascular Endothelial Growth Factor–Silk Fibroin–Chitosan Three-Dimensional Scaffolds for Bone Tissue Engineering and In Vitro Evaluation. J. Craniofac. Surg. 2016, 27, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, M.H.; Kwon, B.J.; Koo, M.A.; Seon, G.M.; Park, J.C. Enhancement of human mesenchymal stem cell infiltration into the electrospun poly(lactic-co-glycolic acid) scaffold by fluid shear stress. Biochem. Biophys. Res. Commun. 2015, 463, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Karr, J.C.; Lauretta, J. In vitro activity of calcium sulfate and hydroxyapatite antifungal disks loaded with amphotericin B or voriconazole in consideration for adjunctive osteomyelitis management. J. Am. Podiatr. Med. Assoc. 2015, 105, 104–110. [Google Scholar] [CrossRef]
- Gu, J.; Wang, T.; Fan, G.; Ma, J.; Hu, W.; Cai, X. Biocompatibility of artificial bone based on vancomycin loaded mesoporous silica nanoparticles and calcium sulfate composites. J. Mater. Sci. Mater. Med. 2016, 27, 1–11. [Google Scholar] [CrossRef]
- Chang, M.P.; Tsung, Y.C.; Hsu, H.C.; Tuan, W.H.; Lai, P.L. Addition of a small amount of glass to improve the degradation behavior of calcium sulfate bioceramic. Ceram. Int. 2015, 41, 1155–1162. [Google Scholar]
- Shen, Y.; Yang, S.; Liu, J.; Xu, H.; Shi, Z.; Lin, Z.; Ying, X.Z.; Guo, P.; Lin, T.; Yan, S.G.; et al. Engineering scaffolds integrated with calcium sulfate and oyster shell for enhanced bone tissue regeneration. ACS Appl. Mater. Interfaces 2014, 6, 12177–12188. [Google Scholar] [CrossRef] [PubMed]
- Shakir, M.; Jolly, R.; Khan, M.S.; Iram, N.E.; Sharma, T.K.; Al-Resayes, S.I. Synthesis and characterization of a nano-hydroxyapatite/chitosan/polyethylene glycol nanocomposite for bone tissue engineering. Polym. Adv. Technol. 2015, 26, 41–48. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.; Ran, J.; Shen, X.; Tong, H. A novel nanocomposite for bone tissue engineering based on chitosan–silk sericin/hydroxyapatite: Biomimetic synthesis and its cytocompatibility. RSC Adv. 2015, 5, 56410–56422. [Google Scholar] [CrossRef]
- Manchon, A.; Alkhraisat, M.; Rueda-Rodriguez, C.; Torres, J.; Prados-Frutos, J.C.; Ewald, A.; López-Cabarcos, E. Silicon calcium phosphate ceramic as novel biomaterial to simulate the bone regenerative properties of autologous bone. J. Biomed. Mater. Res. Part A 2015, 103, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Lowe, B.; Anil, S.; Kim, S.K.; Shim, M.S. Combination of Nano-Hydroxyapatite with Stem Cells for Bone Tissue Engineering. J. Nanosci. Nanotechnol. 2016, 16, 8881–8894. [Google Scholar] [CrossRef]
- Shuai, C.; Feng, P.; Yang, B.; Cao, Y.; Min, A.; Peng, S. Effect of nano-zirconia on the mechanical and biological properties of calcium silicate scaffolds. Int. J. Appl. Ceram. Technol. 2015, 12, 1148–1156. [Google Scholar] [CrossRef]
- Kailasanathan, C.; Selvakumar, N. Comparative study of hydroxyapatite/gelatin composites reinforced with bio-inert ceramic particles. Ceram. Int. 2012, 38, 3569–3582. [Google Scholar] [CrossRef]
- Chan, K.W.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. Polypropylene biocomposites with boron nitride and nanohydroxyapatite reinforcements. Materials 2015, 8, 992–1008. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Li, H.; Li, K.; Lu, J.; Zhang, L. In vitro mineralization of MC3T3-E1 osteoblast-like cells on collagen/nano-hydroxyapatite scaffolds coated carbon/carbon composites. J. Biomed. Mater. Res. Part A 2016, 104, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Sunil, B.R.; Kumar, T.S.S.; Chakkingal, U.; Nandakumar, V.; Doble, M. Friction stir processing of magnesium–nanohydroxyapatite composites with controlled in vitro degradation behavior. Mater. Sci. Eng. C 2014, 39, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, C.; Arroyo, F.; Balanda, C.; Neira, M.; Von Marttens, A.; Caviedes, P.; Rodríguez, J.P.; Urra, C. The effect of the nanoscale structure of nanobioceramics on their in vitro bioactivity and cell differentiation properties. J. Nanomater. 2015, 2015, 526230–526243. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, G.; Yang, S.; Li, J.; Jia, H.; Fang, Z.; Zhang, Q. Effects of in situ and physical mixing on mechanical and bioactive behaviors of nano hydroxyapatite–chitosan scaffolds. J. Biomater. Sci. Polym. Ed. 2011, 22, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, P.; Lin, X.; Liu, Y.; Bian, H.; Zhou, Y.; Gao, C.; Shuai, C. System development, formability quality and microstructure evolution of selective laser-melted magnesium. Virtual Phys. Prototyp. 2016, 11, 173–181. [Google Scholar] [CrossRef]
- Veljovíc, D.; Zalite, I.; Palcevskis, E.; Smiciklas, I.; Petrovíc, R.; Janáckovíc, D. Microwave sintering of fine grained HAP and HAP/TCP bioceramics. Ceram. Int. 2010, 36, 595–603. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, M.; Zhai, W.; Qu, H.; Chang, J. Fabrication and characterization of hydroxyapatite/wollastonite composite bioceramics with controllable properties for hard tissue repair. J. Am. Ceram. Soc. 2011, 94, 99–105. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Gu, Z.; Qin, H.; Li, L.; Liu, J.; Yu, X. In vitro study on the degradation of lithium-doped hydroxyapatite for bone tissue engineering scaffold. Mater. Sci. Eng. C 2016, 66, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Alblas, J.; Hennink, W.E.; Öner, F.C.; Dhert, W.J. Organ printing: The future of bone regeneration? Trends Biotechnol. 2011, 29, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Yang, D.; Yang, Z.; Li, X.; Di, L.Z.; Zhao, H. A study of hydroxyapatite/calcium sulphate bioceramics. Ceram. Int. 2005, 31, 1021–1023. [Google Scholar] [CrossRef]
- Zamanian, A.; Yasaei, M.; Moztarzadeh, F.; Hesaraki, S.; Hafezi, M. Influence of nano-hydroxyapatite addition to calcium hydroxide cement on its Properties. Key Eng. Mater. 2012, 493, 655–660. [Google Scholar] [CrossRef]
- Kuo, S.T.; Wu, H.W.; Tuan, W.H. Resorbable calcium sulfates with tunable degradation rate. J. Asian Ceram. Soc. 2013, 1, 102–107. [Google Scholar] [CrossRef]
- Niu, L.N.; Fang, M.; Jiao, K.; Tang, L.H.; Xiao, Y.H.; Shen, L.J.; Chen, J.H. Tetrapod-like zinc oxide whisker enhancement of resin composite. J. Dent. Res. 2010, 89, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Wang, C.W.; Hsueh, N.S.; Ding, S.J. Improvement of in vitro physicochemical properties and osteogenic activity of calcium sulfate cement for bone repair by dicalcium silicate. J. Alloys Compd. 2014, 585, 25–31. [Google Scholar] [CrossRef]
- Cabanas, M.V.; Rodriguez-Lorenzo, L.M.; Vallet-Regi, M. Setting behavior and in vitro bioactivity of hydroxyapatite/calcium sulfate cements. Chem. Mater. 2002, 14, 3550–3555. [Google Scholar] [CrossRef]
- Kalia, P.; Vizcay-Barrena, G.; Fan, J.P.; Warley, A.; Di Silvio, L.; Huang, J. Nanohydroxyapatite shape and its potential role in bone formation: an analytical study. J. R. Soc. Interface 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Cabrejos-Azama, J.; Alkhraisat, M.H.; Rueda, C.; Torres, J.; Blanco, L.; López-Cabarcos, E. Magnesium substitution in brushite cements for enhanced bone tissue regeneration. Mater. Sci. Eng. C 2014, 43, 403–410. [Google Scholar] [CrossRef] [PubMed]











| Laser Power | Scanning Speed | Spot Diameter | Layer Thickness | Scan Spacing |
|---|---|---|---|---|
| 7.5 W | 100 mm·min−1 | 1.0 mm | 0.1 mm | 3.5 mm |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Yuan, F.; Peng, S.; Xie, H.; Wu, P.; Feng, P.; Gao, C.; Yang, Y.; Guo, W.; Lai, D.; et al. Tunable Degradation Rate and Favorable Bioactivity of Porous Calcium Sulfate Scaffolds by Introducing Nano-Hydroxyapatite. Appl. Sci. 2016, 6, 411. https://doi.org/10.3390/app6120411
Zhou J, Yuan F, Peng S, Xie H, Wu P, Feng P, Gao C, Yang Y, Guo W, Lai D, et al. Tunable Degradation Rate and Favorable Bioactivity of Porous Calcium Sulfate Scaffolds by Introducing Nano-Hydroxyapatite. Applied Sciences. 2016; 6(12):411. https://doi.org/10.3390/app6120411
Chicago/Turabian StyleZhou, Jianhua, Fulai Yuan, Shuping Peng, Hui Xie, Ping Wu, Pei Feng, Chengde Gao, Youwen Yang, Wang Guo, Duan Lai, and et al. 2016. "Tunable Degradation Rate and Favorable Bioactivity of Porous Calcium Sulfate Scaffolds by Introducing Nano-Hydroxyapatite" Applied Sciences 6, no. 12: 411. https://doi.org/10.3390/app6120411
APA StyleZhou, J., Yuan, F., Peng, S., Xie, H., Wu, P., Feng, P., Gao, C., Yang, Y., Guo, W., Lai, D., Zhou, Z., Zhu, X., & Shuai, C. (2016). Tunable Degradation Rate and Favorable Bioactivity of Porous Calcium Sulfate Scaffolds by Introducing Nano-Hydroxyapatite. Applied Sciences, 6(12), 411. https://doi.org/10.3390/app6120411