Photonic Crystal-Based Sensors for Detecting Alcohol Concentration
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of the Polystyrene (PS) Opal Array
2.2. Preparation of the TiO2 Inverse Opal Structure
2.3. Preparation of the Ethanol Concentration Identification Sensor
2.4. Characterizations
3. Results and Discussion
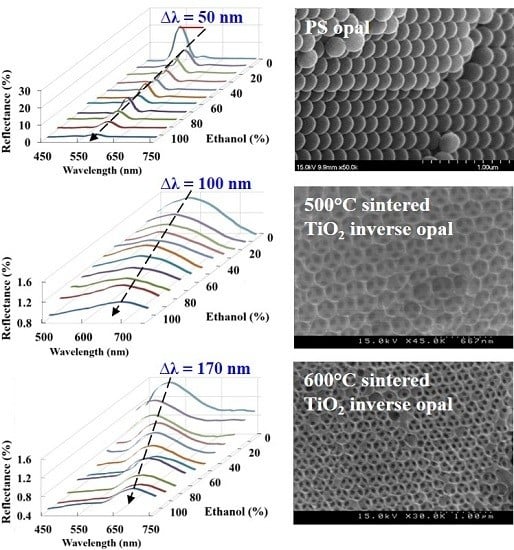
3.1. Characterization of the PS Opal and TiO2 Inverse Opal Structures
3.2. Ethanol Concentration Examined by PS Opal and TiO2 Inverse Opal Structures
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PS | Polystyrene |
| SPR | Surface Plasmon Resonance |
| FCC | Face-centered cubic |
| AFM | Atomic Force Microscopy |
| XRD | X-ray diffraction |
| RIU | Refractive index unit |
References
- Nishijima, Y.; Ueno, K.; Juodkazis, S.; Mizeikis, V.; Misawa, H.; Tanimura, T.; Maeda, K. Inverse silica opal photonic crystals for optical sensing applications. Opt. Express 2007, 15, 12979–12988. [Google Scholar] [CrossRef] [PubMed]
- Holtz, J.H.; Asher, S.A. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 1997, 389, 829–832. [Google Scholar] [CrossRef]
- Li, J.; Zheng, T. A comparison of chemical sensors based on the different ordered inverse opal films. Sens. Actuators B Chem. 2008, 131, 190–195. [Google Scholar] [CrossRef]
- Convertino, A.; Capobianchi, A.; Valentini, A.; Cirillo, E.N. A new approach to organic solvent detection: High-reflectivity Bragg reflectors based on a gold nanoparticle/Teflon-like composite material. Adv. Mater. 2003, 15, 1103–1105. [Google Scholar] [CrossRef]
- Gu, Z.Z.; Horie, R.; Kubo, S.; Yamada, Y.; Fujishima, A.; Sato, O. Fabrication of a metal-coated three-dimensionally ordered macroporous film and its application as a refractive index sensor. Angew. Chem. 2002, 114, 1201–1204. [Google Scholar] [CrossRef]
- Torres-Costa, V.; Agulló-Rueda, F.; Martín-Palma, R.J.; Martínez-Duart, J.M. Porous silicon optical devices for sensing applications. Opt. Mater. 2005, 27, 1084–1087. [Google Scholar] [CrossRef]
- Matsubara, K.; Kawata, S.; Minami, S. Optical chemical sensor based on surface plasmon measurement. Appl. Opt. 1988, 27, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Lu, S.Y.; Chen, S.; Bernards, M.; Jiang, S. Stop band shift based chemical sensing with three-dimensional opal and inverse opal structures. Sens. Actuators B Chem. 2007, 124, 452–458. [Google Scholar] [CrossRef]
- Potyrailo, R.A.; Ghiradella, H.; Vertiatchikh, A.; Dovidenko, K.; Cournoyer, J.R.; Olson, E. Morpho butterfly wing scales demonstrate highly selective vapour response. Nat. Photonics 2007, 1, 123–128. [Google Scholar] [CrossRef]
- Chiappini, A.; Armellini, C.; Chiasera, A.; Ferrari, M.; Fortes, L.; Gonçalves, M.C.; Righini, G.C. An alternative method to obtain direct opal photonic crystal structures. J. Non-Cryst. Solids 2009, 355, 1167–1170. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, J.; Gao, M.; Li, P.; Gao, L.; Heng, L.; Tang, B.Z.; Jiang, L. A visual film sensor based on silole-infiltrated SiO2 inverse opal photonic crystal for detecting organic vapors. J. Mater. Chem. C 2014, 42, 8865–8872. [Google Scholar] [CrossRef]
- Chiappini, A.; Armellini, C.; Carpentiero, A.; Minati, L.; Righini, G.C.; Ferrari, M. Solvent sensitive polymer composite structures. Opt. Mater. 2013, 36, 130–134. [Google Scholar] [CrossRef]
- Wijnhoven, J.E.; Vos, W.L. Preparation of photonic crystals made of air spheres in titania. Science 1998, 281, 802–804. [Google Scholar] [CrossRef]
- Holland, B.T.; Blanford, C.F.; Do, T.; Stein, A. Synthesis of highly ordered, three-dimensional, macroporous structures of amorphous or crystalline inorganic oxides, phosphates, and hybrid composites. Chem. Mater. 1999, 11, 795–805. [Google Scholar] [CrossRef]
- Norell, M.A.; Makovicky, P.; Clark, J.M. Porous silica via colloidal crystallization. Nature 1997, 389, 447–448. [Google Scholar] [CrossRef]
- Tessier, P.M.; Velev, O.D.; Kalambur, A.T.; Rabolt, J.F.; Lenhoff, A.M.; Kaler, E.W. Assembly of gold nanostructured films templated by colloidal crystals and use in surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 2000, 122, 9554–9555. [Google Scholar] [CrossRef]
- Yin, J.S.; Wang, Z.L. Template-assisted self-assembly and cobalt doping of ordered mesoporous titania nanostructures. Adv. Mater. 1999, 11, 469–472. [Google Scholar] [CrossRef]
- Velev, O.D.; Tessier, P.M.; Lenhoff, A.M.; Kaler, E.W. Materials: A class of porous metallic nanostructures. Nature 1999, 401, 548. [Google Scholar] [CrossRef]
- Vlasov, Y.A.; Yao, N.; Norris, D.J. Synthesis of photonic crystals for optical wavelengths from semiconductor quantum dots. Adv. Mater. 1999, 11, 165–169. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Yu, H.H. High refractive index organic-inorganic composites with TiO2 nanocrystal. Thin Solid Films 2013, 529, 195–199. [Google Scholar] [CrossRef]
- Kuo, W.K.; Kuo, G.F.; Lin, S.Y.; Yu, H.H. Fabrication and characterization of artificial miniaturized insect compound eyes for imaging. Bioinspir. Biomim. 2015, 10, 056010. [Google Scholar]
- Kuo, W.K.; Weng, H.P.; Hsu, J.J.; Yu, H.H. A bioinspired color-changing polystyrene microarray as a rapid qualitative sensor for methanol and ethanol. Mater. Chem. Phys. 2016. [Google Scholar] [CrossRef]
- Liu, K.; Schmedake, T.A.; Tsu, R. A comparative study of colloidal silica spheres: Photonic crystals versus Bragg’s law. Phys. Lett. A 2008, 372, 4517–4520. [Google Scholar] [CrossRef]
- Shieh, J.Y.; Kuo, J.Y.; Weng, H.P.; Yu, H.H. Preparation and evaluation of the bioinspired PS/PDMS photochromic films by the self-assembly dip-drawing method. Langmuir 2013, 29, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, G.I.; Waterland, M.R. Opal and inverse opal photonic crystals: Fabrication and characterization. Polyhedron 2007, 26, 356–368. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Zhu, Y.; Chen, H.; Li, Z.; Ding, J.; Chi, Y. Fabrication of anatase titania inverse opal films using polystyrene templates. Superlattices Microstruct. 2006, 40, 155–160. [Google Scholar] [CrossRef]
- Asiah, M.N.; Mamat, M.H.; Khusaimi, Z.; Achoi, M.F.; Abdullah, S.; Rusop, M. Thermal stability and phase transformation of TiO2 nanowires at various temperatures. Microelectron. Eng. 2013, 108, 134–137. [Google Scholar] [CrossRef]
- Amrehn, S.; Wu, X.; Schumacher, C.; Wagner, T. Photonic crystal-based fluid sensors: Toward practical application. Phys. Status Solidi A 2015, 212, 1266–1272. [Google Scholar] [CrossRef]
- Herráez, J.V.; Belda, R. Refractive indices, densities and excess molar volumes of monoalcohols + water. J. Solut. Chem. 2006, 35, 1315–1328. [Google Scholar] [CrossRef]
- Zhao, X.J.; Wang, Z.; Xu, H.Y.; Liang, F.; Zhang, H.Q.; Jin, Q.H. Studies on surface plasmon resonace sensor. Chem. J. Chin. Univ. 1998, 198, 1214–1218. [Google Scholar]
- Park, S.H.; Xia, Y. Assembly of mesoscale particles over large areas and its application in fabricating tunable optical filters. Langmuir 1999, 15, 266–273. [Google Scholar] [CrossRef]
- Di Falco, A.; O’Faolain, L.; Krauss, T.F. Chemical sensing in slotted photonic crystal heterostructure cavities. Appl. Phys. Lett. 2009, 94, 063503. [Google Scholar] [CrossRef]
- Cennamo, N.; Massarotti, D.; Conte, L.; Zeni, L. Low cost sensors based on SPR in a plastic optical fiber for biosensor implementation. Sensors 2011, 11, 11752–11760. [Google Scholar] [CrossRef] [PubMed]
- DeLouise, L.A.; Kou, P.M.; Miller, B.L. Cross-correlation of optical microcavity biosensor response with immobilized enzyme activity. Insights into biosensor sensitivity. Anal. Chem. 2005, 77, 3222–3230. [Google Scholar] [PubMed]
- Roper, D.K. Determining surface plasmon resonance response factors for deposition onto three-dimensional surfaces. Chem. Eng. Sci. 2007, 62, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.C.; Chen, S.Y.; Liu, D.M. Structural evolution and optical properties of TiO2 thin films prepared by thermal oxidation of sputtered Ti films. J. Appl. Phys. 2000, 88, 4628–4633. [Google Scholar] [CrossRef]








| Ethanol Concentration (%) (v/v0) | Refractive Index (by Abbe Refractometer, at 26 ± 0.5 °C) | PS Opal Structure | TiO2 Inverse Opal Structure Sintered Temperature | |
|---|---|---|---|---|
| λ (nm) | λ (nm)(500°C) | λ (nm)(600°C) | ||
| 100 | 1.3601 | 580 | 670 | 670 |
| 90 | 1.3625 | 590 | 630 | 680 |
| 80 | 1.3623 | 590 | 620 | 670 |
| 70 | 1.3616 | 590 | 590 | 650 |
| 60 | 1.3598 | 580 | 580 | 630 |
| 50 | 1.3571 | 580 | 590 | 620 |
| 40 | 1.3536 | 580 | 580 | 600 |
| 30 | 1.3488 | 570 | 590 | 580 |
| 20 | 1.3429 | 570 | 580 | 560 |
| 10 | 1.3375 | 560 | 580 | 540 |
| 0 | 1.3324 | 540 | 570 | 510 |
| ∆λ (λmax − λmin) | - | 50 | 100 | 170 |
| Ethanol Concentration (v/v0) | Refractive Index from Table 1 | Average Refractive Index (na) from Equation (4) | The Wavelength (λ) from Table 1 |
|---|---|---|---|
| 80% | 1.3623 | 1.7391 | 670 (nm) |
| 10% | 1.3375 | 1.7248 | 540 (nm) |
| - | ∆n = 0.0248 | ∆na = 0.0143 | ∆λ = 130 (nm) |
| Sensitivity (S) | 5242 nm/RIU | 9090 nm/RIU | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, W.-K.; Weng, H.-P.; Hsu, J.-J.; Yu, H.H. Photonic Crystal-Based Sensors for Detecting Alcohol Concentration. Appl. Sci. 2016, 6, 67. https://doi.org/10.3390/app6030067
Kuo W-K, Weng H-P, Hsu J-J, Yu HH. Photonic Crystal-Based Sensors for Detecting Alcohol Concentration. Applied Sciences. 2016; 6(3):67. https://doi.org/10.3390/app6030067
Chicago/Turabian StyleKuo, Wen-Kai, Hsueh-Ping Weng, Jyun-Jheng Hsu, and Hsin Her Yu. 2016. "Photonic Crystal-Based Sensors for Detecting Alcohol Concentration" Applied Sciences 6, no. 3: 67. https://doi.org/10.3390/app6030067
APA StyleKuo, W. -K., Weng, H. -P., Hsu, J. -J., & Yu, H. H. (2016). Photonic Crystal-Based Sensors for Detecting Alcohol Concentration. Applied Sciences, 6(3), 67. https://doi.org/10.3390/app6030067