Atomic Layer Deposition TiO2 Films and TiO2/SiNx Stacks Applied for Silicon Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Characterization of ALD TiO2 Thin Films
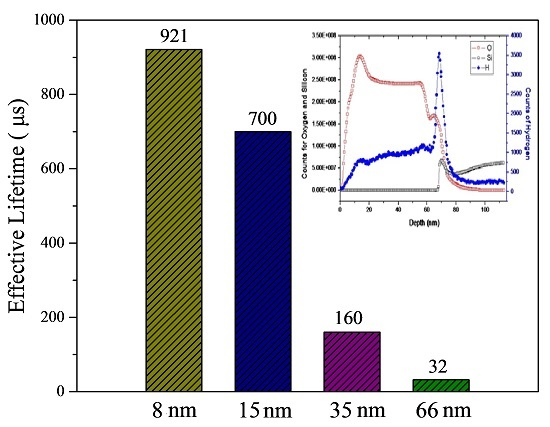
3.2. Silicon Surface Passivation of ALD TiO2
3.3. Reflectivity and Surface Passivation of TiO2/SiNx Stacks
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Green, M.A. The path to 25% silicon solar sell efficiency: History of silicon cell evolution. Prog. Photovoltaics Res. Appl. 2009, 17, 183–189. [Google Scholar] [CrossRef]
- Aberle, A.G. Surface passivation of crystalline silicon solar cells: A review. Prog. Photovoltaics Res. Appl. 2000, 8, 473–487. [Google Scholar] [CrossRef]
- Werner, F.; Veith, B.; Tiba, V.; Poodt, P.; Roozeboom, F.; Brendel, R.; Schmidt, J. Very low surface recombination velocities on p- and n-type c-Si by ultrafast spatial atomic layer deposition of aluminum oxide. Appl. Phys. Lett. 2010, 97, 162103. [Google Scholar] [CrossRef]
- Ge, J.; Tang, M.; Wong, J.; Zhang, Z.; Dippell, T.; Doerr, M.; Hohn, O.; Huber, M.; Wohlfart, P.; Aberle, A.G.; et al. Excellent silicon surface passivation achieved by industrial inductively coupled plasma deposited hydrogenated intrinsic amorphous silicon suboxide. In. J. Photoenergy 2014, 2014. [Google Scholar] [CrossRef]
- Breitenstein, L.; Richter, A.; Hermle, M.; Warta, W. Impact of wet-chemical cleaning on the passivation quality of Al2O3 layers. In Proceedings of the 37th IEEE Photovoltaic Specialists Conference, Seattle, WA, USA, 19–24 June 2011; pp. 1400–1404.
- Schmidt, J.; Veith, B.; Werner, F.; Zielke, D.; Brendel, R. Silicon surface passivation by ultrathin Al2O3 films and Al2O3/SiNx stacks. In Proceedings of the 35th IEEE Photovoltaic Specialists Conference, Honolulu, HI, USA, 20–25 June 2010; pp. 885–890.
- Hoex, B.; Schmidt, J.; Pohl, P.; Van de Sanden, M.C.M.; Kessels, W.M.M. Silicon surface passivation by atomic layer deposited Al2O3. J. Appl. Phys. 2008, 104. [Google Scholar] [CrossRef]
- Vermang, B.; Werner, F.; Stals, W.; Lorenz, A.; Rothschild, A.; John, J.; Poortmans, J.; Mertens, R.; Gortzen, R.; Poodt, P.; et al. Spatially-separated atomic layer deposition of Al2O3, a new option for high-throughput Si solar cell passivation. Prog. Photovoltaics Res. Appl. 2010, 19, 733–739. [Google Scholar] [CrossRef]
- Lee, B.G.; Li, S.; Von Gastrow, G.; Yli-Koski, M.; Savin, H.; Malinen, V.; Skarp, J.; Choi, S.; Branz, H.M. Excellent passivation and low reflectivity with atomic layer deposited bilayer coatings for n-type silicon solar cells. Thin Solid Films 2014, 550, 541–544. [Google Scholar] [CrossRef]
- Davis, K.O.; Jiang, K.; Habermann, D.; Schoenfeld, W.V. Tailoring the optical properties of APCVD titanium oxide films for all-oxide multi-layer anti-reflection coatings. IEEE J. Photovoltaics 2015, 5, 1265–1270. [Google Scholar] [CrossRef]
- Suh, D. Stacked and nanolaminated Al2O3/TiO2 for surface passivation and encapsulation of silicon. Phys. Status Solidi (RRL) 2015, 9, 344–347. [Google Scholar] [CrossRef]
- Benner, F.; Jordan, P.M.; Richter, C.; Simon, D.K.; Dirnstorfer, I.; Knaut, M.; Bartha, J.W.; Mikolajick, T. Atomic layer deposited high-κ nanolaminates for silicon surface passivation. J. Vac. Sci. Technol. B 2014, 32. [Google Scholar] [CrossRef]
- Dirnstorfer, I.; Chohan, T.; Jordan, P.M.; Knaut, M.; Simon, D.K.; Bartha, J.W.; Mikolajick, T. Al2O3-TiO2 nanolaminates for conductive silicon surface passivation. IEEE J. Photovoltaics 2016, 6, 86–91. [Google Scholar] [CrossRef]
- Melskens, J.; Van de Loo, B.W.H.; Macco, B.; Vos, M.F.J.; Palmans, J.; Smit, S.; Kessels, W.M.M. Concepts and prospects of passivating contacts for crystalline silicon solar cells. In Proceedings of the 42nd IEEE Photovoltaic Specialists Conference, New Orleans, LA, USA, 14–19 June 2015; pp. 1–6.
- Richards, B.S. Comparison of TiO2 and other dielectric coatings for buried contact solar cells: A review. Prog. Photovoltaics Res. Appl. 2004, 12, 253–281. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Lin, F.-R.; Lin, T.C.; Chen, C.-H.; Pei, Z. Low-temperature, chemically grown titanium oxide thin films with a high hole tunneling rate for Si solar cells. Energies 2016, 9. [Google Scholar] [CrossRef]
- Rohatgi, A.; Doshi, P.; Moschner, J.; Lauinger, T.; Aberle, A.G.; Ruby, D.S. Compressive study of rapid low-cost silicon surface passivation technologies. IEEE Tran. Electron Devices 2000, 47, 987–993. [Google Scholar] [CrossRef]
- Doeswijk, L.M.; De Moor, H.H.C.; Blank, D.H.A.; Rogalla, H. Passivating TiO2 coatings for silicon solar cells by pulsed laser deposition. Appl. Phys. A 1999, 69. [Google Scholar] [CrossRef]
- Thomson, A.F.; Lynn, S.Z.; McIntosh, K.R. Passivation of silicon by negatively charged TiO2. In Proceedings of the 25th EUPVSEC, Valencia, Spain, 6–10 September 2010; pp. 1146–1153.
- Thomson, A.F.; McIntosh, K.R. Light-enhanced surface passivation of TiO2-coated silicon. Prog. Photovoltaics Res. Appl. 2012, 20, 343–349. [Google Scholar] [CrossRef]
- Yu, I.-S.; Wang, Y.-W.; Cheng, H.-E.; Yang, Z.-P.; Lin, C.-T. Surface passivation and antireflection behavior of ALD TiO2 on n-type silicon for solar cells. Int. J. Photoenergy 2013, 2013. [Google Scholar] [CrossRef]
- Liao, B.; Hoex, B.; Aberle, A.G.; Chi, D.; Bhatia, C.S. Excellent c-Si surface passivation by low-temperature atomic layer deposited titanium oxide. Appl. Phys. Lett. 2014, 104. [Google Scholar] [CrossRef]
- Liao, B.; Hoex, B.; Shetty, K.D.; Basu, P.K.; Bhatia, C.B. Passivation of boron-doped industrial silicon emitters by thermal atomic layer deposited titanium oxide. IEEE J. Photovoltaics 2015, 5, 1062–1065. [Google Scholar] [CrossRef]
- Avasthi, S.; McClain, W.E.; Mam, G.; Kahn, A.; Schwartz, J.; Sturm, J.C. Hole-blocking titanium-oxide/silicon heterojunction and its application to photovoltaics. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, P.; Bi, Q.; Weber, K. Silicon heterojunction solar cells with electron selective TiOx contact. Sol. Energy Mater. Sol. Cells 2016, 150, 32–38. [Google Scholar] [CrossRef]
- Yang, X.; Bi, Q.; Ali, H.; Davis, K.O.; Schoenfeld, W.V.; Weber, K. High performance TiO2-based electron-selective contacts for crystalline silicon solar cells. Adv. Mater. 2016, 28, 5891–5897. [Google Scholar] [CrossRef] [PubMed]
- Gad, K.M.; Vossing, D.; Rimin, A.; Rayner, B.; Reindl, L.M.; Mohney, S.E.; Kasemann, M. Ultrathin titanium dioxide nanolayers by atomic layer deposition for surface passivation of crystalline silicon. IEEE J. Photovoltaics 2016, 6, 649–653. [Google Scholar] [CrossRef]
- Cheng, H.-E.; Chen, C.-C. Morphological and photoelectrochemical properties of ALD TiO2 films. J. Electrochem. Soc. 2008, 155, D604–D607. [Google Scholar] [CrossRef]
- McIntosh, K.R.; Guo, J.-H.; Abbott, M.D.; Bardos, R.A. Calibration of the WCT-100 photoconductance instrument at low conductance. Prog. Photovoltaics Rese. Appl. 2008, 16, 279–287. [Google Scholar] [CrossRef]
- Sinton, R.A.; Cuevas, A.; Stuckings, M. Quasi-steady-state photoconductance: A new method for solar cell material and device characterization. In Proceedings of the 25th IEEE Photovoltaic Specialists Conference, Washington, DC, USA, 13–17 May 1996; pp. 457–460.
- Won, D.-J.; Wang, C.-H.; Jang, H.-K.; Choi, D.-J. Effect of thermally induced anatase-to-rutile phase transition in MOCVD growth TiO2 films on structural and optical properties. Appl. Phys. A 2001, 73, 595–600. [Google Scholar] [CrossRef]
- Yu, I.-S.; Chang, I.-H.; Cheng, H.-E.; Lin, Y.-S. Surface passivation of c-Si by atomic layer deposition TiO2 thin films deposited at low temperature. In Proceedings of the 40th IEEE Photovoltaic Specialists Conference, Denver, CO, USA, 8–13 June 2014; pp. 1271–1274.
- Georgescu, D.; Baia, L.; Ersen, O.; Baia, M.; Simon, S. Experimental assessment of phonon confinement in TiO2 anatase nanocrystallites by Raman spectroscopy. J. Raman Spectrosc. 2012, 43, 876–883. [Google Scholar] [CrossRef]
- Sahoo, S.; Arora, A.K.; Sridharan, V. Raman line shapes of optical phonons of different symmetries in anatase TiO2 nanocrystals. J. Phys. Chem. C 2009, 113, 16927–16933. [Google Scholar] [CrossRef]
- Jellison, G.E.; Boatner, L.A., Jr.; Budai, J.D.; Jeong, B.-S.; Norton, D.P. Spectroscopic ellipsometry of thin film and bulk anatase (TiO2). J. Appl. Phys. 2003, 93, 9537–9541. [Google Scholar] [CrossRef]
- Richards, B.S. Single-material TiO2 double-layer antireflection coatings. Sol. Energy Mater. Sol. Cells 2003, 79, 369–390. [Google Scholar] [CrossRef]
- PV Lighthouse. Available online: http://www.pvlighthouse.com.au/calculators/OPAL%202/OPAL%202.aspx (accessed on 20 May 2015).
- Kane, D.E.; Swanson, R.M. Measurement of the emitter saturation current by a contactless photoconduc tivity decay method. In Proceedings of the 18th IEEE Photovoltaic Specialists Conference, Las Vegas, NV, USA, 21–25 October 1985; pp. 578–583.
- Rahman, M.Z.; Khan, S.I. Advances in surface passivation of c-Si solar cells. Mater. Renew. Sustain. Energy 2012, 1. [Google Scholar] [CrossRef]
- Wan, Y.; McIntosh, K.R.; Thomson, A.F. Characterisation and optimization of PECVD SiNx as an antireflection coating and passivation layer for silicon cells. AIP Adv. 2013, 3. [Google Scholar] [CrossRef]









© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.-P.; Cheng, H.-E.; Chang, I.-H.; Yu, I.-S. Atomic Layer Deposition TiO2 Films and TiO2/SiNx Stacks Applied for Silicon Solar Cells. Appl. Sci. 2016, 6, 233. https://doi.org/10.3390/app6080233
Yang Z-P, Cheng H-E, Chang I-H, Yu I-S. Atomic Layer Deposition TiO2 Films and TiO2/SiNx Stacks Applied for Silicon Solar Cells. Applied Sciences. 2016; 6(8):233. https://doi.org/10.3390/app6080233
Chicago/Turabian StyleYang, Zu-Po, Hsyi-En Cheng, I-Hsuan Chang, and Ing-Song Yu. 2016. "Atomic Layer Deposition TiO2 Films and TiO2/SiNx Stacks Applied for Silicon Solar Cells" Applied Sciences 6, no. 8: 233. https://doi.org/10.3390/app6080233
APA StyleYang, Z. -P., Cheng, H. -E., Chang, I. -H., & Yu, I. -S. (2016). Atomic Layer Deposition TiO2 Films and TiO2/SiNx Stacks Applied for Silicon Solar Cells. Applied Sciences, 6(8), 233. https://doi.org/10.3390/app6080233