Evaluation of Antioxidant and Cytotoxicity Activities of Copper Ferrite (CuFe2O4) and Zinc Ferrite (ZnFe2O4) Nanoparticles Synthesized by Sol-Gel Self-Combustion Method
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of CuFe2O4 and ZnFe2O4 Nanoparticles
2.2. Characterization
2.3. Antioxidant Activity (Evaluation of DPPH Free Radical Scavenging Activity)
2.4. Evaluation of Cytotoxicity Activity
3. Results and Discussion
3.1. X-ray Diffraction (XRD) Analysis
3.2. FTIR Spectra Analysis
3.3. Morphological Analysis
3.4. Magnetic Analysis
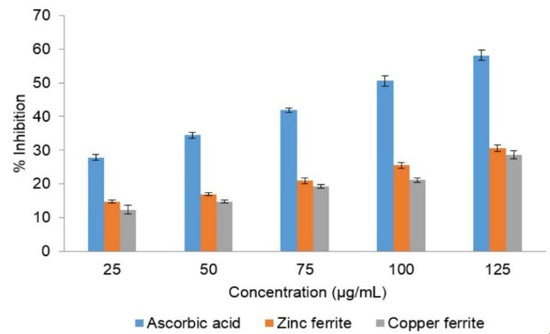
3.5. Antioxidant Activity
3.6. Cytotoxicity Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Perez, J.A.L.; Quintela, M.A.L.; Mira, J.; Rivas, J.; Charles, S.W. Advances in the preparation of magnetic nanoparticles by the microemulsion method. J. Phys. Chem. B 1997, 101, 8045–8047. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Z.J. Size-dependent superparamagnetic properties of MgFe2O4 spinel ferrite nanocrystallites. Appl. Phys. Lett. 1998, 73, 3156–3158. [Google Scholar] [CrossRef]
- Tang, Z.X.; Sorensen, C.M.; Klabunde, K.J.; Hadjipanayis, G.C. Preparation of Mn ferrite fine particles from aqueous solution. J. Colloid Interface Sci. 1991, 146, 38–46. [Google Scholar] [CrossRef]
- Seip, C.T.; Carpenter, E.E.; O’Connor, C.J.; John, V.T.; Li, S. Magnetic properties of a series of ferrite nanoparticles synthesized in reverse micelles. IEEE Trans. Magn. 1998, 34, 1111–1113. [Google Scholar] [CrossRef]
- Hochepied, J.F.; Bonville, P.; Pileni, M.P. Nonstoichiometric zinc ferrite nanocrystals: Syntheses and unusual magnetic properties. J. Phys. Chem. B 2000, 104, 905–912. [Google Scholar] [CrossRef]
- Liu, C.; Zou, B.; Rondinone, A.J.; Zhang, Z.J. Reverse micelle synthesis and characterization of superparamagnetic MnFe2O4 spinel ferrite nanocrystallites. J. Phys. Chem. B 2000, 104, 1141–1145. [Google Scholar] [CrossRef]
- Raghavender, A.T.; Kulkarni, R.G.; Jadhav, K.M. Magnetic properties of mixed cobalt-aluminum ferrite nanoparticles. Chin. J. Phys. 2010, 48, 512–522. [Google Scholar]
- Rajath Varma, P.C.; Manna, R.S.; Banerjee, D.; Varma, M.R.; Suresh, K.G.; Nigam, A.K. Magnetic properties of CoFe2O4 synthesized by solid state, citrate precursor and polymerized complex methods: A comparative study. J. Alloys Comp. 2008, 453, 298–303. [Google Scholar] [CrossRef]
- Kahn, M.L.; Zhang, Z.J. Synthesis and magnetic properties of CoFe2O4 spinel ferrite nanoparticles doped with lanthanide ions. Appl. Phys. Lett. 2001, 78, 3651–3653. [Google Scholar] [CrossRef]
- Mohapatra, M.; Anand, S. Synthesis and applications of nano-structured iron oxides/hydroxides—A review. Int. J. Eng. Sci. Technol. 2010, 2, 127–146. [Google Scholar] [CrossRef]
- Zak, T.; Cosovic, V.; Cosovic, A.; David, B.; Talijan, N.; Zivkovic, D. Formation of magnetic microstructure of the nanosized NiFe2O4 synthesized via solid-state reaction. Sci. Sinter. 2012, 44, 103–112. [Google Scholar] [CrossRef]
- Standley, K.J. Oxide Magnetic Materials, 2nd ed.; Oxford University Press: Oxford, UK, 1972. [Google Scholar]
- Yattinahalli, S.; Kapatkar, S.B.; Ayachit, N.H.; Mathad, S.N. Synthesis and structural haracterization of nanosized nickel ferrite. Int. J. Self-Propag. High-Temp. Synth. 2013, 22, 147–150. [Google Scholar] [CrossRef]
- Safarik, I.; Horska, K.; Pospiskova, K.; Safarikova, M. Magnetic techniques for the detection and determination of xenobiotics and cells in water. Anal. Bioanal. Chem. 2012, 404, 1257–1273. [Google Scholar] [CrossRef] [PubMed]
- Pospiskova, K.; Safarik, I.; Sebela, M.; Kuncova, G. Magnetic particles-based biosensor for biogenic amines using an optical oxygen sensor as a transducer. Microchim. Acta 2013, 180, 311–318. [Google Scholar] [CrossRef]
- Cui, X.; Belo, S.; Krüger, D.; Yan, Y.; de Rosales, R.T.M.; Jauregui-Osoro, M.; Ye, H.; Su, S.; Mathe, D.; Kovacs, N.; et al. Aluminium hydroxide stabilised MnFe2O4 and Fe3O4 nanoparticles as dual-modality contrasts agent for MRI and PET imaging. Biomaterials 2014, 35, 5840–5846. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Feng, J.; Xu, X.; Zhang, M. Synthesis and characterizations of spinel MnFe2O4 nanorod by seed-hydrothermal route. J. Alloys Compd. 2010, 491, 258–263. [Google Scholar] [CrossRef]
- Shanmugavela, T.; Gokul Rajb, S.; Ramesh Kumarc, G.; Rajarajana, G. Synthesis and structural analysis of nanocrystalline MnFe2O4. Phys. Procedia 2014, 54, 159–163. [Google Scholar] [CrossRef]
- Zaki, T.; Saed, D.; Aman, D.; Younis, S.A.; Moustafa, Y.M. Synthesis and characterization of MFe2O4 sulfur nanoadsorbents. J. Sol-Gel Sci. Technol. 2013, 65, 269–276. [Google Scholar] [CrossRef]
- Kanagesan, S.; Hashim, M.; Tamilselvan, S.; Alitheen, N.B.; Ismail, I.; Syazwan, M.; ZuikimiI, M.M.M. Sol-gel auto-combustion synthesis of cobalt ferrite and it’s cytotoxicity properties. Digest J. Nanomater. Biostruct. 2013, 8, 1601–1610. [Google Scholar]
- Qi, X.; Zhou, J.; Yue, Z.; Gui, Z.; Li, L. Permeability and microstructure of manganese modified lithium ferrite prepared by sol-gel auto-combustion method. Mater. Sci. Eng. B 2003, 99, 278–281. [Google Scholar] [CrossRef]
- Yan, S.; Ling, W.; Zhou, E. Rapid synthesis of Mn0.65Zn0.35Fe2O4/SiO2 homogeneous nanocomposites by modified sol-gel auto-combustion method. J. Cryst. Growth. 2004, 273, 226–233. [Google Scholar] [CrossRef]
- Mangalaraja, R.V.; Anathakmar, S.; Manohar, P.; Gnanam, F.D.; Awana, M. Characterization of Mn0.8Zn0.2Fe2O4 synthesized by flash combustion technique. Mater. Sci. Eng. A 2004, 367, 301–305. [Google Scholar] [CrossRef]
- Mangalaraja, R.V.; Anathakmar, S.; Manohar, P.; Gnanam, F.D. Magnetic, electrical and dielectric behaviour of Ni0.8Zn0.2Fe2O4 prepared through flash combustion technique. J. Magn. Magn. Mater. 2002, 253, 56–64. [Google Scholar] [CrossRef]
- Sileo, E.E.; Jacobo, S.E. Gadolinium-nickel ferrites prepared from metal citrates precursors. Physica B 2004, 354, 241–245. [Google Scholar] [CrossRef]
- Bujoreanu, V.M.; Diamandescu, L.; Brezeanu, M. On the structure of manganese ferrite powder prepared by coprecipitation from MnO2 and FeSO4·7H2O. Mater. Lett. 2000, 46, 169–174. [Google Scholar] [CrossRef]
- Upadhyay, R.V.; Davies, K.J.; Wells, S.; Charles, S.W. Preparation and characterization of ultra-fine MnFe2O4 and MnxFe1−xFe2O4 spinel systems: I. particles. J. Magn. Magn. Mater. 1994, 132, 249–257. [Google Scholar] [CrossRef]
- Shi, Y.; Ding, J.; Liu, X.; Wang, J. NiFe2O4 ultrafine particles prepared by co-precipitation/mechanical alloying. J. Magn. Magn. Mater. 1999, 205, 249–254. [Google Scholar] [CrossRef]
- Azadmanjiria, J.; Seyyed Ebrahimib, S.A.; Salehania, H.K. Magnetic properties of nanosize NiFe2O4 particles synthesized by sol-gel auto combustion method. Ceram. Int. 2007, 33, 1623–1625. [Google Scholar] [CrossRef]
- Kanagesan, S.; Hashim, M.; Tamilselvan, S.; Alitheen, N.B.; Ismail, I.; Ismail, M.A.N.; Bahmanrokh, G.; Rahman, M.M. Characteristics and cytotoxicity of magnetic nanoparticles on breast cancer cells. J. Optoelectron. Adv. Mater. 2014, 6, 41–50. [Google Scholar]
- Karimi, Z.; Karimi, L.; Shokrollahi, H. Nano-magnetic particles used in biomedicine: Core and coating materials. Mater. Sci. Eng. C 2013, 33, 2465–2475. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Konar, S.; Pathak, A.; Dasgupta, S.; Das Gupta, S. Effect of functionalized magnetic MnFe2O4 nanoparticles on fibrillation of human serum albumin. J. Phys. Chem. B 2014, 118, 11667–11676. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Sinniah, U.R.; Akhtar, M.S. In vitro pharmacological activities and GC-MS analysis of different solvent extracts of Lantana camara leaves collected from tropical region of Malaysia. Evid. Based Complement. Alternat. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Akhtar, M.S.; Mohanty, S.K.; Sinniah, U.R. Synthesis and characterization of silver nanoparticles using fruit extract of Momordica cymbalaria and assessment of their in vitro antimicrobial, antioxidant and cytotoxicity activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 151, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Nath, B.C.; Phukon, P.; Dolui, S.K. Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Colloids Surf. B 2013, 101, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Swamy, M.K.; Umar, A.; Al Sahli, A.A. Biosynthesis and characterization of silver nanoparticles from methanol leaf extract of Cassia didymobotyra and assessment of their antioxidant and antibacterial activities. J. Nanosci. Nanotechnol. 2015, 15, 9818–9823. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.; Devi, K.S.P.; Banerjee, R.; Maiti, T.K.; Pramanik, P.; Dhara, D. Thermal and pH responsive polymer-tethered multifunctional magnetic nanoparticles for targeted delivery of anticancer drug. ACS Appl. Mater. Inter. 2013, 5, 3884–3893. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, S.; Zhao, D.; Hun, F.H.; Weng, L.; Liu, H. Cytotoxicity, permeability, and inflammation of metal oxide nanoparticles in human cardiac microvascular endothelial cells: Cytotoxicity, permeability, and inflammation of metal oxide nanoparticles. Cell Biol. Toxicol. 2011, 27, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Khanna, L.; Verma, N.K. Synthesis, characterization and in vitro cytotoxicity study of calcium ferrite nanoparticles. Mater. Sci. Semicond. Process. 2013, 16, 1842–1848. [Google Scholar] [CrossRef]
- Kanagesan, S.; Hashim, M.; Tamilselvan, S.; Alitheen, N.B.; Ismail, I.; Bahmanrokh, G. Cytotoxic effect of nanocrystalline MgFe2O4 particles for cancer cure. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- Chandradass, J.; Jadhav, A.H.; Kim, K.H.; Kim, H. Influence of processing methodology on the structural and magnetic behavior of MgFe2O4 nanopowders. J. Alloys Compd. 2012, 517, 164–169. [Google Scholar] [CrossRef]
- Dolci, S.; Domenici, V.; Duce, C.; Tiné, M.R.; Ierardi, V.; Valbusa, U.; Jaglicic, Z.; Boni, A.; Gemmi, M.; Pampaloni, G. Ultrasmall superparamagnetic iron oxide nanoparticles with titanium-N,N-dialkylcarbamato coating. Mater. Res. Express 2014, 1. [Google Scholar] [CrossRef]
- Krehula, S.; Music, S. Influence of Mn-dopant on the properties of α-FeOOH particles precipitated in highly alkaline media. J. Alloys Compd. 2006, 426, 327–334. [Google Scholar] [CrossRef]
- Iacob, M. Sonochemical synthesis of hematite nanoparticles, chemistry journal of moldova. Gen. Ind. Ecol. Chem. 2015, 10, 46–51. [Google Scholar]
- Tahar, L.B.; Smiri, L.S.; Artus, M.; Joudrier, A.; Herbst, F.; Vaulay, M.J.; Ammar, S.; Fievet, F. Characterization and magnetic properties of Sm- and Gd-substituted CoFe2O4 nanoparticles prepared by forced hydrolysis in polyol. Mater. Res. Bull. 2007, 42, 1888–1896. [Google Scholar] [CrossRef]
- Deng, H.; Chen, H.; Li, H. Synthesis of crystal MFe2O4 (M = Mg, Cu, Ni) microspheres. Mater. Chem. Phys. 2007, 101, 509–513. [Google Scholar] [CrossRef]
- Smit, J.; Wijn, H.P.J. Ferrites Physical Properties of Ferromagnetic Oxides in Relation to Their Technical Applications; Wiley: New York, NY, USA, 1959. [Google Scholar]
- Kovacic, P.; Somanathan, R. Nanoparticles: Toxicity, radicals, electron transfer, and antioxidants. Methods Mol. Biol. 2013, 1028, 15–35. [Google Scholar] [PubMed]
- Das, D.; Nath, B.C.; Phukon, P.; Dolui, S.K. Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity. Colloids Surf. B 2013, 111, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.S.J.P.; Saikia, J.P.; Samdarshi, S.K.; Konwar, B.K. Investigation of antioxidant property of iron oxide particles by 1′-1′ diphenylpicryl-hydrazyle (DPPH) method. J. Magn. Magn. Mater. 2009, 321, 3621–3623. [Google Scholar] [CrossRef]
- Covaliu, C.I.; Matei, C.; Litescu, S.; Eremia, S.A.M.; Stanica, N.; Diamandescu, L.; Ianculescu, A.; Jitaru, I.; Berger, D. Radical scavenger properties of oxide nanoparticles stabilized with biopolymer matrix. Rev. Mater. Plast. 2010, 47, 5–10. [Google Scholar]
- Saikia, J.P.; Paul, S.; Konwar, B.K.; Samdarshi, S.K. Nickel oxide nanoparticles: A novel antioxidant. Colloids Surf. B 2010, 78, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Javorina, A.K.; Schrand, A.M.; Duhart, H.M.; Ali, S.F.; Schlager, J.J. The interaction of manganese nanoparticles with PC-12 cells induces dopamine depletion. Toxicol. Sci. 2006, 92, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Alhadlaq, H.A.; Akhtar, M.J.; Ahamed, M. Zinc ferrite nanoparticle-induced cytotoxicity and oxidative stress in different human cells. Cell Biosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Sudipta, K.M.; Jayanta, K.; Balasubramanya, S. The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulata leaf extract. Appl. Nanosci. 2015, 5, 73–81. [Google Scholar] [CrossRef]
- Tomitaka, A.; Hirukawa, A.; Yamada, T.; Morishita, S.; Takemura, Y. Biocompatibility of various ferrite nanoparticles evaluated by in vitro cytotoxicity assays using HeLa cells. J. Magn. Magn. Mater. 2009, 321, 1482–1484. [Google Scholar] [CrossRef]
- Leung, K.C.F.; Wang, Y.X.J. Mn–Fe nanowires towards cell labeling and magnetic resonance imaging. In Nanowires Science and Technology; Nicoleta, L., Ed.; In Tech Open Access Publisher: Hong Kong, China, 2010. [Google Scholar]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Saptarshi, S.R.; Feltis, B.N.; Wright, P.F.; Lopata, A.L. Investigating the immunomodulatory nature of zinc oxide nanoparticles at sub-cytotoxic levels in vitro and after intranasal instillation in vivo. J. Nanobiotechnol. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Akhavan, O.; Meidanchi, A.; Laurent, S.; Mahmoudi, M. Hyperthermia-induced protein corona improves the therapeutic effects of zinc ferrite spinel-graphene sheets against cancer. RSC Adv. 2014, 4, 62557–62565. [Google Scholar] [CrossRef]
- Akhavan, O.; Meidanchi, A.; Ghaderi, E.; Khoei, S. Zinc ferrite spinel-graphene in magneto-photothermal therapy of cancer. J. Mater. Chem. B 2014, 2, 3306–3314. [Google Scholar] [CrossRef]
- Meidanchi, A.; Akhavan, O.; Khoei, S.; Shokri, A.A.; Hajikarimi, Z.; Khansari, N. ZnFe2O4 nanoparticles as radiosensitizers in radiotherapy of human prostate cancer cells. Mater. Sci. Eng. C 2015, 46, 394–399. [Google Scholar] [CrossRef] [PubMed]







© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanagesan, S.; Hashim, M.; AB Aziz, S.; Ismail, I.; Tamilselvan, S.; Alitheen, N.B.; Swamy, M.K.; Purna Chandra Rao, B. Evaluation of Antioxidant and Cytotoxicity Activities of Copper Ferrite (CuFe2O4) and Zinc Ferrite (ZnFe2O4) Nanoparticles Synthesized by Sol-Gel Self-Combustion Method. Appl. Sci. 2016, 6, 184. https://doi.org/10.3390/app6090184
Kanagesan S, Hashim M, AB Aziz S, Ismail I, Tamilselvan S, Alitheen NB, Swamy MK, Purna Chandra Rao B. Evaluation of Antioxidant and Cytotoxicity Activities of Copper Ferrite (CuFe2O4) and Zinc Ferrite (ZnFe2O4) Nanoparticles Synthesized by Sol-Gel Self-Combustion Method. Applied Sciences. 2016; 6(9):184. https://doi.org/10.3390/app6090184
Chicago/Turabian StyleKanagesan, Samikannu, Mansor Hashim, Sidek AB Aziz, Ismayadi Ismail, Subramani Tamilselvan, Noorjahan Banu Alitheen, Mallappa Kumara Swamy, and Bandaru Purna Chandra Rao. 2016. "Evaluation of Antioxidant and Cytotoxicity Activities of Copper Ferrite (CuFe2O4) and Zinc Ferrite (ZnFe2O4) Nanoparticles Synthesized by Sol-Gel Self-Combustion Method" Applied Sciences 6, no. 9: 184. https://doi.org/10.3390/app6090184
APA StyleKanagesan, S., Hashim, M., AB Aziz, S., Ismail, I., Tamilselvan, S., Alitheen, N. B., Swamy, M. K., & Purna Chandra Rao, B. (2016). Evaluation of Antioxidant and Cytotoxicity Activities of Copper Ferrite (CuFe2O4) and Zinc Ferrite (ZnFe2O4) Nanoparticles Synthesized by Sol-Gel Self-Combustion Method. Applied Sciences, 6(9), 184. https://doi.org/10.3390/app6090184