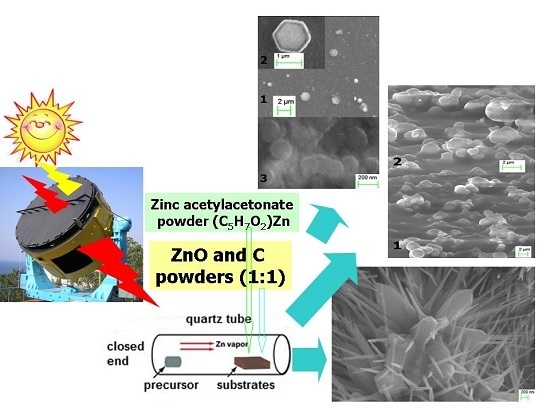
Solar Explosive Evaporation Growth of ZnO Nanostructures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Characterization
3. Results
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lashkarev, G.V.; Karpyna, V.A.; Lazorenko, V.I.; Ievtushenko, A.I.; Shtepliuk, I.I.; Khranovskyy, V.D. Properties of zinc oxide at low and moderate temperatures. Low Temp. Phys. 2011, 37, 226–234. [Google Scholar] [CrossRef]
- Abbasi, H.Y.; Habib, A.; Tanveer, M. Synthesis and characterization of nanostructures of ZnO and ZnO/Graphene composites for the application in hybrid solar cells. Alloys Compd. 2017, 690, 21–26. [Google Scholar] [CrossRef]
- Willander, M.; Nur, O.; Zhao, Q.X.; Yang, L.L.; Lorenz, M.; Cao, B.Q.; Zuniga, P.J.; Czekalla, C.; Zimmermann, G.; Grundmann, M.; et al. Zinc oxide nanorod based photonic devices: Recent progress in growth, light emitting diodes and lasers. Nanotechnology 2009, 20, 332001. [Google Scholar] [CrossRef] [PubMed]
- Djurisic, A.B.; Chen, X.; Leung, Y.H.; Ng, A.M.C. ZnO nanostructures: growth, properties and applications. J. Mater. Chem. 2012, 22, 6526–6535. [Google Scholar] [CrossRef]
- Khranovskyy, V.; Yakimova, R. Morphology engineering of ZnO nanostructures. Phys. B 2012, 407, 1533–1537. [Google Scholar] [CrossRef]
- Khranovskyy, V.; Tsiaoussis, I.; Yazdi, G.R.; Hultman, L.; Yakimova, R. Heteroepitaxial ZnO nano hexagons on p-type SiC. Cryst. Growth 2010, 312, 327–332. [Google Scholar] [CrossRef]
- Khranovskyy, V.; Yakimova, R.; Karlsson, F.; Syed, A.S.; Holtz, P.-O.; Urgessa, Z.N.; Oluwafemi, O.S.; Botha, J.R. Comparative PL study of individual ZnO nanorods, grown by APMOCVD and CBD techniques. Phys. B 2012, 407, 1538–1542. [Google Scholar] [CrossRef]
- Khranovskyy, V.; Lazorenko, V.; Lashkarev, G.; Yakimova, R. Luminescence anisotropy of ZnO microrods. J. Lumin. 2012, 132, 2643–2647. [Google Scholar] [CrossRef]
- Christien, F.; Ferchaud, E.; Nowakowski, P.; Allart, M. The Use of Electron Probe MicroAnalysis to Determine the Thickness of Thin Films in Materials Science. In X-ray Spectroscopy; Shatendra, K.S., Ed.; INTECH: Rijeka, Croatia, 2012. [Google Scholar]
- Ievtushenko, A.; Karpyna, V.; Lashkarev, G.; Lazorenko, V.; Baturin, V.; Karpenko, A.; Lunika, M.; Dan’ko, A. Multilayered ZnO Films of Improved Quality Deposited by Magnetron Sputtering. Acta Phys. Polonica A 2008, 114, 1131–1137. [Google Scholar] [CrossRef]
- Habibi, M.H.; Rahmati, M.H. Fabrication and characterization of ZnO@CdS core–shell nanostructure using acetate precursors: XRD, FESEM, DRS, FTIR studies and effects of cadmium ion concentration on band gap. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 133, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Damen, T.C.; Porto, S.P.S.; Tell, B. Raman Effect in Zinc Oxide. Phys. Rev. 1966, 142, 570–574. [Google Scholar] [CrossRef]
- Wang, J.B.; Zhong, H.M.; Li, Z.F.; Lu, W. Raman study of N+-implanted ZnO. Appl. Phys. Lett. 2006, 88, 101913. [Google Scholar] [CrossRef]
- Ievtushenko, A.I.; Karpyna, V.A.; Lazorenko, V.I.; Lashkarev, G.V.; Khranovskyy, V.D.; Baturin, V.A.; Karpenko, O.Y.; Lunika, M.M.; Avramenko, K.A.; Strelchuk, V.V.; et al. High quality ZnO films deposited by radio-frequency magnetron sputtering using layer by layer growth method. Thin Solid Films 2010, 518, 4529–4532. [Google Scholar] [CrossRef]
- Serrano, J.; Manjon, F.G.; Romero, A.H.; Widulle, F.; Lauck, R.; Cardona, M. Dispersive Phonon Linewidths: The E2 Phonons of ZnO. Phys. Rev. Lett. 2003, 90, 055510. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, F.; Nickel, N.H. Resonant Raman scattering in hydrogen and nitrogen doped ZnO. Appl. Phys. Lett. 2007, 91, 111903. [Google Scholar] [CrossRef]
- Kaschner, A.; Haboeck, U.; Strassburg, M.; Strassburg, M.; Kaczmarczyk, G.; Hoffmann, A.; Thomsen, C.; Zeuner, A.; Alves, H.R.; Hoffmann, D.M.; et al. Nitrogen-related local vibrational modes in ZnO:N. Appl. Phys. Lett. 2002, 80, 1909. [Google Scholar]
- Ievtushenko, A.I.; Lashkarev, G.V.; Lazorenko, V.I.; Karpyna, V.A.; Dusheyko, M.G.; Tkach, V.M.; Kosyachenko, L.A.; Sklyarchuk, V.M.; Sklyarchuk, O.F.; Avramenko, K.A.; et al. Effect of nitrogen doping on photoresponsivity of ZnO films. Phys. Stat. Sol. 2010, 207, 1746–1750. [Google Scholar] [CrossRef]
- Artús, L.; Cuscó, R.; Alarcón-Lladó, E.; González-Díaz, G.; Mártil, I.; Jiménez, J.; Wang, B.; Callahan, M. Isotopic study of the nitrogen-related modes in N+-implanted ZnO. App. Phys. Lett. 2007, 90, 181911. [Google Scholar] [CrossRef]
- Ievtushenko, A.I.; Lashkaryov, G.V.; Strelchuk, V.V.; Lazorenko, V.I.; Klochkov, L.O.; Lytvyn, O.S.; Tkach, V.M.; Romanyuk, A.S.; Avramenko, K.A.; Bykov, O.I.; et al. Structural transformations in films of ZnO under their alloying with nitrogen and Al-N Co-alloying. Metallofiz. Noveishie Tekhnol. 2011, 33, 243–254. [Google Scholar]
- Bundesmann, C.; Ashkenov, N.; Schubert, M.; Spemann, D.; Butz, T.; Kaidashev, E.; Lorenz, M.; Grundmann, M. Raman scattering in ZnO thin films doped with Fe, Sb, Al, Ga, and Li. Appl. Phys. Lett. 2003, 83, 1974–1976. [Google Scholar] [CrossRef]
- Tzolov, M.; Tzenov, N.; Dimova-Malinovska, D.; Kalitzova, M.; Pizzuto, C.; Vitali, G.; Zollo, G.; Ivanov, I. Vibrational properties and structure of undoped and Al-doped ZnO films deposited by RF magnetron sputtering. Thin Solid Films 2000, 379, 28–36. [Google Scholar] [CrossRef]
- Manjón, F.J.; Marí, B.; Serrano, J.; Romero, A.H. Silent Raman modes in zinc oxide and related nitrides. Appl. Phys. 2005, 97, 053516. [Google Scholar] [CrossRef]
- Dijken, A.; Meulenkamp, E.A.; Vanmaekelbergh, D.; Meijerink, A. The Kinetics of the Radiative and Nonradiative Processes in Nanocrystalline ZnO Particles upon Photoexcitation. Phys. Chem. B 2000, 104, 1715–1723. [Google Scholar] [CrossRef]
- Ahn, C.H.; Kim, Y.Y.; Kim, D.C.; Mohanta, S.K.; Cho, H.K. A comparative analysis of deep level emission in ZnO layers deposited by various methods. Appl. Phys. 2009, 105, 013502. [Google Scholar] [CrossRef]
- Kaur, G.; Mitra, A.; Yadav, K.L. Pulsed laser deposited Al-doped ZnO thin films for optical applications. Prog. Nat. Sci. Mater. Int. 2015, 25, 12–21. [Google Scholar] [CrossRef]






| ZnO Morphology | Crystal Planes (h k l) | 2θ(h k l) (deg.) | XRD FWHM (deg.) | Integral Intensity of XRD (h k l) (a.u.) |
|---|---|---|---|---|
| Needles | (002) | 34.36 | 0.18 | 157.2 |
| (101) | 36.21 | 0.36 | 3.7 | |
| (102) | 47.51 | 0.24 | 5.1 | |
| (103) | 62.87 | 0.33 | 24.6 | |
| Spheres | (002) | 34.09 | 0.48 | 18.9 |
| (103) | 63.12 | 0.9 | 23.9 | |
| (112) | 67.63 | 0.99 | 18.71 | |
| Hexagons | (002) | 34.17 | 0.12 | 0.45 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ievtushenko, A.; Tkach, V.; Strelchuk, V.; Petrosian, L.; Kolomys, O.; Kutsay, O.; Garashchenko, V.; Olifan, O.; Korichev, S.; Lashkarev, G.; et al. Solar Explosive Evaporation Growth of ZnO Nanostructures. Appl. Sci. 2017, 7, 383. https://doi.org/10.3390/app7040383
Ievtushenko A, Tkach V, Strelchuk V, Petrosian L, Kolomys O, Kutsay O, Garashchenko V, Olifan O, Korichev S, Lashkarev G, et al. Solar Explosive Evaporation Growth of ZnO Nanostructures. Applied Sciences. 2017; 7(4):383. https://doi.org/10.3390/app7040383
Chicago/Turabian StyleIevtushenko, Arsenii, Vasily Tkach, Victor Strelchuk, Larisa Petrosian, Oleksander Kolomys, Oleksander Kutsay, Viktor Garashchenko, Olena Olifan, Sergiy Korichev, Georgii Lashkarev, and et al. 2017. "Solar Explosive Evaporation Growth of ZnO Nanostructures" Applied Sciences 7, no. 4: 383. https://doi.org/10.3390/app7040383
APA StyleIevtushenko, A., Tkach, V., Strelchuk, V., Petrosian, L., Kolomys, O., Kutsay, O., Garashchenko, V., Olifan, O., Korichev, S., Lashkarev, G., & Khranovskyy, V. (2017). Solar Explosive Evaporation Growth of ZnO Nanostructures. Applied Sciences, 7(4), 383. https://doi.org/10.3390/app7040383