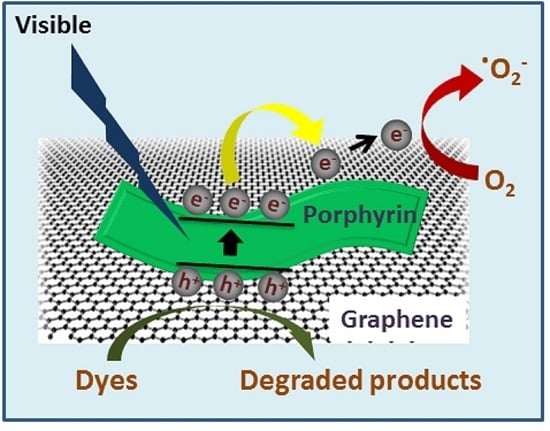
Arginine-Mediated Self-Assembly of Porphyrin on Graphene: A Photocatalyst for Degradation of Dyes
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of H2TCPP
2.3. Arginine-Mediated Self-Assembly of TCPP on the Graphene Surface
2.4. Photocatlytic Investigation
2.5. Characterization
3. Results
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Drain, C.M.; Varotto, A.; Radivojevic, I. Self-organized porphyrinic materials. Chem. Rev. 2009, 109, 1630–1658. [Google Scholar] [CrossRef] [PubMed]
- Auwärter, W.; Écija, D.; Klappenberger, F.; Barth, J.V. Porphyrins at interfaces. Nat. Chem. 2015, 7, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Hupp, J.T. Porphyrin-containing molecular squares: Design and applications. Chem. Rev. 2006, 250, 1710–1723. [Google Scholar] [CrossRef]
- Sakakibara, K.; Hill, J.P.; Ariga, K. Thin-Film-Based Nanoarchitectures for Soft Matter: Controlled Assemblies into Two-Dimensional Worlds. Small 2011, 7, 1288–1308. [Google Scholar] [CrossRef] [PubMed]
- Würthner, F.; Kaiser, T.E.; Saha-Möller, C.R. J-Aggregates: From Serendipitous Discovery to Supramolecular Engineering of Functional Dye Materials. Angew. Chem. Int. Ed. 2011, 50, 3376–3410. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, J.; Zhang, Z.G.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Elemans, J.A.; van Hameren, R.; Nolte, R.J.; Rowan, A.E. Molecular Materials by Self-Assembly of Porphyrins, Phthalocyanines, and Perylenes. Adv. Mater. 2006, 18, 1251–1266. [Google Scholar] [CrossRef]
- Hoeben, F.J.; Jonkheijm, P.; Meijer, E.; Schenning, A.P. About supramolecular assemblies of π-conjugated systems. Chem. Rev. 2005, 105, 1491–1546. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.M. Toward self-organization and complex matter. Science 2002, 295, 2400–2403. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Qi, L. Surfactant-assisted, shape-controlled synthesis of gold nanocrystals. Nanoscale 2011, 3, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zhu, W.; Yang, H.; An, Q.; Tao, C.A.; Li, W.; Cui, J.; Li, Z.; Li, G. Facile Fabrication of Stimuli-Responsive Polymer Capsules with Gated Pores and Tunable Shell Thickness and Composite. Angew. Chem. Int. Ed. 2011, 123, 5049–5053. [Google Scholar] [CrossRef]
- Jang, J.; Oh, J.H. Facile Fabrication of Photochromic Dye–Conducting Polymer Core–Shell Nanomaterials and Their Photoluminescence. Adv. Mater. 2003, 15, 977–980. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Pellegrino, T.; Manna, L. Synthesis, properties and perspectives of hybrid nanocrystal structures. Chem. Soc. Rev. 2006, 35, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Medforth, C.J.; Shelnutt, J.A. Porphyrin nanotubes by ionic self-assembly. J. Am. Chem. Soc. 2004, 126, 15954–15955. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Chen, P.; Ma, W.; Liu, M. Morphology-dependent supramolecular photocatalytic performance of porphyrin nanoassemblies: From molecule to artificial supramolecular nanoantenna. J. Mater. Chem. 2012, 22, 20243–20249. [Google Scholar] [CrossRef]
- Lee, S.J.; Hupp, J.T.; Nguyen, S.T. Growth of narrowly dispersed porphyrin nanowires and their hierarchical assembly into macroscopic columns. J. Am. Chem. Soc. 2008, 130, 9632–9633. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Malliakas, C.D.; Kanatzidis, M.G.; Hupp, J.T.; Nguyen, S.T. Amphiphilic porphyrin nanocrystals: Morphology tuning and hierarchical assembly. Adv. Mater. 2008, 20, 3543–3549. [Google Scholar] [CrossRef]
- Gong, X.; Milic, T.; Xu, C.; Batteas, J.D.; Drain, C.M. Preparation and characterization of porphyrin nanoparticles. J. Am. Chem. Soc. 2002, 124, 14290–14291. [Google Scholar] [CrossRef] [PubMed]
- Hasobe, T.; Oki, H.; Sandanayaka, A.S.D.; Hideyuki, M. Sonication-assisted supramolecular nanorods of meso-diaryl-substituted porphyrins. Chem. Commun. 2008, 724–726. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Ma, C.; Li, X.; Jiang, J. Morphology-Controlled Self-Assembled Nanostructures of 5, 15-Di [4-(5-acetylsulfanylpentyloxy) phenyl] porphyrin Derivatives. Effect of Metal—Ligand Coordination Bonding on Tuning the Intermolecular Interaction. J. Am. Chem. Soc. 2008, 130, 17044–17052. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.S.; Guo, Y.G.; Liang, H.P.; Wan, L.J.; Jiang, L. Three-dimensional self-organization of supramolecular self-assembled porphyrin hollow hexagonal nanoprisms. J. Am. Chem. Soc. 2005, 127, 17090–17095. [Google Scholar] [CrossRef] [PubMed]
- Medforth, C.J.; Wang, Z.; Martin, K.E.; Song, Y.; Jacobsen, J.L.; Shelnutt, J.A. Self-assembled porphyrin nanostructures. Chem. Commun. 2009, 47, 7261–7277. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, A.; Huang, Z.H.; Wang, L.N.; Kang, F. Porphyrin-Based Nanostructures for Photocatalytic Applications. Nanomaterials 2016, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, Z.H.; Yue, M.; Kang, F. Integrating porphyrin nanoparticles into a 2D graphene matrix for free-standing nanohybrid films with enhanced visible-light photocatalytic activity. Nanoscale 2014, 6, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Nayak, S.K.; Mallampalli, S.; Patra, A. Surfactant-Assisted Porphyrin Based Hierarchical Nano/Micro Assemblies and Their Efficient Photocatalytic Behavior. ACS Appl. Mater. Interfaces 2014, 6, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, Z.; Zhang, R.; Bai, F.; Wu, H.; Haddad, R.; Fan, H. Interfacial self-assembly driven formation of hierarchically structured nanocrystals with photocatalytic activity. ACS Nano 2014, 8, 827–833. [Google Scholar] [CrossRef] [PubMed]
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. Arginine-induced porphyrin-based self-assembled nanostructures for photocatalytic applications under simulated sunlight irradiation. Photochem. Photobiol. Sci. 2017, 16, 151–154. [Google Scholar] [CrossRef] [PubMed]
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Revaprasadu, N.; Bhosale, S.V. Fabrication of a Graphene@TiO2@ Porphyrin hybrid material and its photocatalytic properties under simulated sunlight irradiation. ChemistrySelect 2017, 2, 3329–3333. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-graphene composite as a high performance photocatalyst. ACS Nano 2009, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Fang, X. Synthesis and Development of Graphene–Inorganic Semiconductor Nanocomposites. Chem. Rev. 2015, 115, 8294–8343. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Han, C.; Jiang, B.; Iocozzia, J.; He, C.; Shi, D.; Jiang, T.; Lin, Z. Graphene-based materials with tailored nanostructures for energy conversion and storage. Mater. Sci. Eng. R Rep. 2016, 102, 1–72. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Yu, W.; Huang, Z.; Zhou, F.; Song, J.; Song, Y.; Long, L.; Cifuentes, M.P.; Humphrey, M.G.; Zhang, L. Covalent functionalization of reduced graphene oxide with porphyrin by means of diazonium chemistry for nonlinear optical performance. Sci. Rep. 2016, 6, 23325. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Song, W.; Kim, S.; Kang, M.A.; Myung, S.; Lee, S.S.; Lim, J.; An, K.S. Tunable functionalization of graphene nanosheets for graphene-organic hybrid photodetectors. Nanotechnology 2016, 27, 075709. [Google Scholar] [CrossRef] [PubMed]
- Stylianakis, M.; Konios, D.; Kakavelakis, G.; Charalambidis, G.; Stratakis, E.; Coutsolelos, A.; Kymakis, E.; Anastasiadis, S. Efficient ternary organic photovoltaics incorporating a graphene-based porphyrin molecule as a universal electron cascade material. Nanoscale 2015, 7, 17827–17835. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Garnica, M.; Bischoff, F.; Ducke, J.; Bocquet, M.L.; Batzill, M.; Auwärter, W.; Barth, J.V. Fusing tetrapyrroles to graphene edges by surface-assisted covalent coupling. Nat. Chem. 2017, 9, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Chen, P.; Liu, M. One-dimensional porphyrin nanoassemblies assisted via graphene oxide: Sheetlike functional surfactant and enhanced photocatalytic behaviors. ACS Appl. Mater. Interfaces 2013, 5, 5336–5345. [Google Scholar] [CrossRef] [PubMed]
- La, D.D.; Rananaware, A.; Salimimarand, M.; Bhosale, S.V. Well–dispersed assembled porphyrin nanorods on graphene for the enhanced photocatalytic performance. ChemistrySelect 2016, 1, 4430–4434. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, B.; Zhou, L.; Liu, P.; Deng, W. Synthesis of Novel Porphyrin Derivatives with Mesogenic Properties. Syn. Commun. 2015, 45, 2730–2739. [Google Scholar] [CrossRef]
- La, M.; Duc, D.; Bhargava, S.; Bhosale, S.V. Improved and a simple approach for mass production of graphene nanoplatelets material. ChemistrySelect 2016, 1, 949–952. [Google Scholar] [CrossRef]
- Sun, J.; Meng, D.; Jiang, S.; Wu, G.; Yan, S.; Geng, J.; Huang, Y. Multiple-bilayered RGO–porphyrin films: From preparation to application in photoelectrochemical cells. J. Mater. Chem. 2012, 22, 18879–18886. [Google Scholar] [CrossRef]
- Kano, H.; Kobayashi, T. Time-resolved fluorescence and absorption spectroscopies of porphyrin J-aggregates. J. Chem. Phys. 2002, 116, 184–195. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, H.L.; Zhang, Q.; Peng, J.; Li, J.; Zhai, M.; Yu, Z.Z. Facile synthesis of well-dispersed graphene by γ-ray induced reduction of graphene oxide. J. Mater. Chem. 2012, 22, 13064–13069. [Google Scholar] [CrossRef]
- Afzal, S.; Daoud, W.A.; Langford, S.J. Self-cleaning cotton by porphyrin-sensitized visible-light photocatalysis. J. Mater. Chem. 2012, 22, 4083–4088. [Google Scholar] [CrossRef]
- Wan, J.; Wang, H.; Wu, Z.; Shun, Y.C.; Zheng, X.; Phillips, D.L. Resonance Raman spectroscopy and density functional theory calculation study of photodecay dynamics of tetra (4-carboxyphenyl) porphyrin. Phys. Chem. Chem. Phys. 2011, 13, 10183–10190. [Google Scholar] [CrossRef] [PubMed]
- McConnell, I.; Li, G.; Brudvig, G.W. Energy conversion in natural and artificial photosynthesis. Chem. Biol. 2010, 17, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Barber, J. Photosynthetic energy conversion: Natural and artificial. Chem. Soc. Rev. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- La, D.D.; Rananaware, A.; Thi, H.P.N.; Jones, L.; Bhosale, S.V. Fabrication of a TiO2@ porphyrin nanofiber hybrid material: A highly efficient photocatalyst under simulated sunlight irradiation. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 015009. [Google Scholar]
- Meadows, P.J.; Dujardin, E.; Hall, S.R.; Mann, S. Template-directed synthesis of silica-coated J-aggregate nanotapes. Chem. Commun. 2005, 29, 3688–3690. [Google Scholar] [CrossRef] [PubMed]
- Kano, H.; Saito, T.; Kobayashi, T. Dynamic intensity borrowing in porphyrin J-aggregates revealed by sub-5-fs spectroscopy. J. Phys. Chem. B 2001, 105, 413–419. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Zhang, X.; Ou, X.; Zhang, X. One-step growth of organic single-crystal p–n nano-heterojunctions with enhanced visible-light photocatalytic activity. Chem. Commun. 2013, 49, 9200–9202. [Google Scholar] [CrossRef] [PubMed]
- La, D.D.; Ramanathan, R.; Rananaware, A.; Bansal, V.; Bhosale, S.V. Nanostructured charge transfer complex of CuTCNQF 4 for efficient photo-removal of hexavalent chromium. RSC Adv. 2016, 6, 33931–33936. [Google Scholar] [CrossRef]
- Li, D.; Dong, W.; Sun, S.; Shi, Z.; Feng, S. Photocatalytic degradation of acid chrome blue K with porphyrin-sensitized TiO2 under visible light. J. Phys. Chem. C 2008, 112, 14878–14882. [Google Scholar] [CrossRef]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La, D.D.; Hangarge, R.V.; V. Bhosale, S.; Ninh, H.D.; Jones, L.A.; Bhosale, S.V. Arginine-Mediated Self-Assembly of Porphyrin on Graphene: A Photocatalyst for Degradation of Dyes. Appl. Sci. 2017, 7, 643. https://doi.org/10.3390/app7060643
La DD, Hangarge RV, V. Bhosale S, Ninh HD, Jones LA, Bhosale SV. Arginine-Mediated Self-Assembly of Porphyrin on Graphene: A Photocatalyst for Degradation of Dyes. Applied Sciences. 2017; 7(6):643. https://doi.org/10.3390/app7060643
Chicago/Turabian StyleLa, Duong Duc, Rahul V. Hangarge, Sidhanath V. Bhosale, Ha Duc Ninh, Lathe A. Jones, and Sheshanath V. Bhosale. 2017. "Arginine-Mediated Self-Assembly of Porphyrin on Graphene: A Photocatalyst for Degradation of Dyes" Applied Sciences 7, no. 6: 643. https://doi.org/10.3390/app7060643
APA StyleLa, D. D., Hangarge, R. V., V. Bhosale, S., Ninh, H. D., Jones, L. A., & Bhosale, S. V. (2017). Arginine-Mediated Self-Assembly of Porphyrin on Graphene: A Photocatalyst for Degradation of Dyes. Applied Sciences, 7(6), 643. https://doi.org/10.3390/app7060643