Dynamic Performance Analysis for an Absorption Chiller under Different Working Conditions
Abstract
:Featured Application
Abstract
1. Introduction
2. Principle
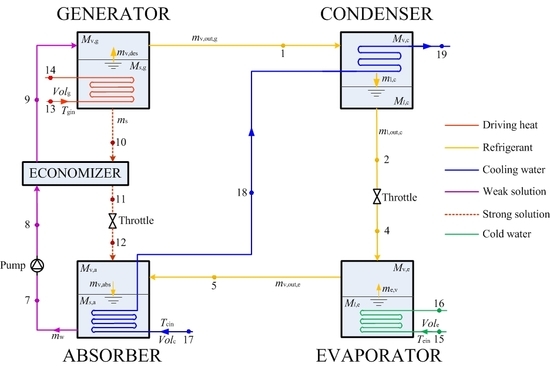
3. Modelling
3.1. Assumptions
- (1)
- There is no heat exchange between the components and the ambient;
- (2)
- The pressures in the generator and the absorber are equal to those in the condenser and the evaporator, respectively.
- (3)
- The solutions leaving the generator and the absorber, and the refrigerants at the outlet of the condenser and the evaporator are saturated;
- (4)
- The transport delays in the fluid cycles are neglected;
- (5)
- The enthalpies of the fluids at the inlet and outlet of the throttle valves are equal.
3.2. Mass and Species Conservation
3.2.1. Generator
3.2.2. Condenser
3.3. Energy Conservation
3.3.1. Generator
3.3.2. Condenser
3.4. Efficiency
3.5. Validation of Model
4. Dynamic Performance Analysis
4.1. Generator Inlet Temperature (Tgin)
4.1.1. Dynamic Response Process
4.1.2. Different Step Change of Generator Inlet Temperature (ΔTgin)
4.2. Different Cooling Water Inlet Temperature (Tcin)
4.3. Different Evaporator Inlet Temperature (Tein)
5. Application Analysis
6. Conclusions
- (1)
- Compared with the step change of the generator inlet temperature ΔTgin = 3 °C, the time required to reach a new steady-state (relaxation time) increases by 11.11%–35.42% when ΔTgin increases from 5 °C to 15 °C.
- (2)
- The relaxation time grows with the rise of the cooling water inlet temperature Tcin, and it increases by 5.65% when Tcin changes from 25 °C to 35 °C.
- (3)
- Reducing evaporator inlet temperature Tein can lengthen the relaxation time, which rises by 3.95% when Tein decreases from 25 °C to 10 °C.
- (4)
- The control strategy considering the AC dynamic performance under different working conditions is beneficial for the real-time operation and control of the whole system.
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| A | bottom area, m2 |
| Cd | discharge coefficient |
| Cp | specific heat, kJ/(kg·K) |
| g | gravitational acceleration, m/s2 |
| H | vertical distance, m |
| h | specific enthalpy, kJ/kg |
| i | time, s |
| M | mass storage, kg |
| MCp | product of thermal storage mass and its specific heat, kJ/K |
| m | mass flow rate, kg/s |
| p | pressure, Pa |
| Pa | state parameters |
| Q | heat exchange rate, kW |
| S | section area, m2 |
| T | temperature, °C |
| V | volume, m3 |
| Vol | volumetric flow rate, m3/h |
| x | mass concentration of LiBr |
| UA | product of heat transfer coefficient and area, kW/K |
| z | fluid storage height, m |
| Greek symbols | |
| ρ | density, kg/m3 |
| ζ | resistance coefficient |
| ΔTgin | increased generator inlet temperature, °C |
| ΔQ | relative heat exchange rate difference, % |
| Abbreviations | |
| AC | absorption chiller |
| COP | coefficient of performance |
| LMTD | logarithmic mean temperature difference, °C |
| Subscripts | |
| a | absorber |
| c | condenser |
| con | container |
| e | evaporator |
| ext | external |
| des | desorbed refrigerant |
| g | generator |
| hx | heat exchanger |
| i | time |
| int | internal |
| l | refrigerant liquid |
| load | process plant |
| out | outlet |
| ref | reference |
| s | strong solution |
| st | steady-state |
| v | vapor |
| w | weak solution |
| wa | water |
| 1, 2···19 | points |
References
- Tsinghua University Building Energy Saving Research Center. 2009 Annual Report on China Building Energy Efficiency; China Architecture and Building Press: Beijing, China, 2009. (In Chinese) [Google Scholar]
- Ding, G.L. Recent developments in simulation techniques for vapour-compression refrigeration systems. Int. J. Refrig. 2007, 30, 1119–1133. [Google Scholar] [CrossRef]
- Wang, Q.; He, W.; Liu, Y.; Liang, G.; Li, J.; Han, X.; Chen, G. Vapor compression multifunctional heat pumps in China: A review of configurations and operational modes. Renew. Sustain. Energy Rev. 2012, 16, 6522–6538. [Google Scholar] [CrossRef]
- Wu, W.; Shi, W.X.; Li, X.T.; Wang, B.L. Air source absorption heat pump in district heating: Applicability analysis and improvement options. Energy Convers. Manag. 2015, 96, 197–207. [Google Scholar] [CrossRef]
- Lubis, A.; Jeong, J.; Saito, K.; Giannetti, N.; Yabase, H.; Alhamid, M.I. Solar-assisted single-double-effect absorption chiller for use in Asian tropical climates. Renew. Energy 2016, 99, 825–835. [Google Scholar] [CrossRef]
- Wu, W.; Wang, B.L.; Shang, S.; Shi, W.X.; Li, X.T. Experimental investigation on NH3-H2O compression-assisted absorption heat pump (CAHP) for low temperature heating in colder conditions. Int. J. Refrig. 2016, 67, 109–124. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.L.; Wu, W.; Li, X.T.; Shi, W.X. Performance analysis of an absorption-compression hybrid refrigeration system recovering condensation heat for generation. Appl. Therm. Eng. 2016, 108, 54–65. [Google Scholar] [CrossRef]
- Grossman, G.; Zaltash, A. ABSIM-modular simulation of advanced absorption systems. Int. J. Refrig. 2001, 24, 531–543. [Google Scholar] [CrossRef]
- Monné, C.; Alonso, S.; Palacín, F.; Serra, L. Monitoring and simulation of an existing solar powered absorption cooling system in Zaragoza (Spain). Appl. Therm. Eng. 2011, 31, 28–35. [Google Scholar] [CrossRef]
- Somers, C.; Mortazavi, A.; Hwang, Y.; Radermacher, R.; Rodgers, P.; Al-Hashimi, S. Modeling water/lithium bromide absorption chillers in ASPEN Plus. Appl. Energy 2011, 88, 4197–4205. [Google Scholar] [CrossRef]
- Ali, A.H.H.; Noeres, P.; Pollerberg, C. Performance assessment of an integrated free cooling and solar powered single-effect lithium bromide-water absorption chiller. Sol. Energy 2008, 82, 1021–1030. [Google Scholar] [CrossRef]
- Gutiérrez-Urueta, G.; Rodríguez, P.; Ziegler, F.; Lecuona, A.; Rodríguez-Hidalgo, M.D.C. Extension of the characteristic equation to absorption chillers with adiabatic absorbers. Int. J. Refrig. 2012, 35, 709–718. [Google Scholar] [CrossRef] [Green Version]
- Zamora, M.; Bourouis, M.; Coronas, A.; Vallès, M. Part-load characteristics of a new ammonia/lithium nitrate absorption chiller. Int. J. Refrig. 2015, 56, 43–51. [Google Scholar] [CrossRef]
- Wu, W.; Shi, W.; Wang, J.; Wang, B.L.; Li, X.T. Experimental investigation on NH3-H2O compression-assisted absorption heat pump (CAHP) for low temperature heating under lower driving sources. Appl. Energy 2016, 176, 258–271. [Google Scholar] [CrossRef]
- Ochoa, A.A.V.; Dutra, J.C.C.; Henríquez, J.R.G.; Dos Santos, C.A.C. Dynamic study of a single effect absorption chiller using the pair LiBr/H2O. Energy Convers. Manag. 2016, 108, 30–42. [Google Scholar] [CrossRef]
- Kohlenbach, P.; Ziegler, F. A dynamic simulation model for transient absorption chiller performance. Part I: The model. Int. J. Refrig. 2008, 31, 217–225. [Google Scholar] [CrossRef]
- Shin, Y.; Seo, J.A.; Cho, H.W.; Nam, S.C.; Jeong, J.H. Simulation of dynamics and control of a double-effect LiBr-H2O absorption chiller. Appl. Therm. Eng. 2009, 29, 2718–2725. [Google Scholar] [CrossRef]
- Kim, B.; Park, J. Dynamic simulation of a single-effect ammonia-water absorption chiller. Int. J. Refrig. 2007, 30, 535–545. [Google Scholar] [CrossRef]
- Cai, W.; Sen, M.; Paolucci, S. Dynamic modeling of an absorption refrigeration system using ionic liquids. In Proceedings of the ASME 2007 International Mechanical Engineering Congress and Exposition, Seattle, WA, USA, 11–15 November 2007; pp. 227–236. [Google Scholar]
- De la Calle, A.; Roca, L.; Bonilla, J.; Palenzuela, P. Dynamic modeling and simulation of a double-effect absorption heat pump. Int. J. Refrig. 2016, 72, 171–191. [Google Scholar] [CrossRef]
- Mansouri, R.; Bourouis, M.; Bellagi, A. Experimental investigations and modelling of a small capacity diffusion-absorption chiller in dynamic mode. Appl. Therm. Eng. 2017, 113, 653–662. [Google Scholar] [CrossRef]
- Myat, A.; Thu, K.; Kim, Y.D.; Chakraborty, A.; Chun, W.G.; Ng, K.C. A second law analysis and entropy generation minimization of an absorption chiller. Appl. Therm. Eng. 2011, 31, 2405–2413. [Google Scholar] [CrossRef]
- Butz, D.; Stephan, K. Dynamic behavior of an absorption heat pump. Int. J. Refrig. 1989, 12, 204–212. [Google Scholar] [CrossRef]
- Jeong, S.; Kang, B.H.; Karng, S.W. Dynamic simulation of an absorption heat pump for recovering low grade waste heat. Appl. Therm. Eng. 1998, 18, 1–12. [Google Scholar] [CrossRef]
- Kohlenbach, P.; Ziegler, F. A dynamic simulation model for transient absorption chiller performance. Part II: Numerical results and experimental verification. Int. J. Refrig. 2008, 31, 226–233. [Google Scholar] [CrossRef]
- Evola, G.; Le Pierrès, N.; Boudehenn, F.; Papillon, P. Proposal and validation of a model for the dynamic simulation of a solar-assisted single-stage LiBr/water absorption chiller. Int. J. Refrig. 2013, 36, 1015–1028. [Google Scholar] [CrossRef]
- Ochoa, A.A.V.; Dutra, J.C.C.; Henríquez, J.R.G.; Dos Santos, C.A.C.; Rohatgi, J. The influence of the overall heat transfer coefficients in the dynamic behavior of a single effect absorption chiller using the pair LiBr/H2O. Energy Convers. Manag. 2017, 136, 270–282. [Google Scholar] [CrossRef]
- Yin, H.; Qu, M.; Archer, D.H. Model based experimental performance analysis of a microscale LiBr-H2O steam-driven double-effect absorption Chiller. Appl. Therm. Eng. 2010, 30, 1741–1750. [Google Scholar] [CrossRef]
- Iranmanesh, A.; Mehrabian, M.A. Dynamic simulation of a single-effect LiBr-H2O absorption refrigeration cycle considering the effects of thermal masses. Energy Build. 2013, 60, 47–59. [Google Scholar] [CrossRef]














| Parameter | Unit | Value | Parameter | Unit | Value |
|---|---|---|---|---|---|
| Ac | m2 | 0.10 | Sg | m2 | 0.0002 |
| Ag | m2 | 0.16 | UAext,a | kW/K | 4.78 |
| Cd | / | 0.61 | UAext,c | kW/K | 9.32 |
| Cpwa | kJ/(kg·K) | 4.19 | UAext,e | kW/K | 4.24 |
| g | m2/s | 9.81 | UAext,g | kW/K | 2.39 |
| Hc | m | 0 | UAint,a | kW/K | 5.97 |
| Hg | m | 0 | UAint,c | kW/K | 9.72 |
| MCpa,con | kJ/K | 58.31 | UAint,e | kW/K | 4.62 |
| MCpc,con | kJ/K | 32.82 | UAint,g | kW/K | 1.79 |
| MCpe,con | kJ/K | 37.83 | UAxs | kW/K | 0.042 |
| MCpg,con | kJ/K | 58.31 | Va | m3 | 0.024 |
| MCphx,a | kJ/K | 4.94 | Vc | m3 | 0.014 |
| MCphx,c | kJ/K | 3.04 | Ve | m3 | 0.024 |
| MCphx,e | kJ/K | 2.66 | Vg | m3 | 0.024 |
| MCphx,g | kJ/K | 2.66 | Volc | m3/h | 2.2 |
| Ml,c,0 | kg | 0.04 | Vole | m3/h | 1.34 |
| Ms,a,0 | kg | 0.09 | Volg | m3/h | 0.82 |
| Sc | m2 | 0.00002 | Volw | m3/h | 0.0666 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Shang, S.; Li, X.; Wang, B.; Wu, W.; Shi, W. Dynamic Performance Analysis for an Absorption Chiller under Different Working Conditions. Appl. Sci. 2017, 7, 797. https://doi.org/10.3390/app7080797
Wang J, Shang S, Li X, Wang B, Wu W, Shi W. Dynamic Performance Analysis for an Absorption Chiller under Different Working Conditions. Applied Sciences. 2017; 7(8):797. https://doi.org/10.3390/app7080797
Chicago/Turabian StyleWang, Jian, Sheng Shang, Xianting Li, Baolong Wang, Wei Wu, and Wenxing Shi. 2017. "Dynamic Performance Analysis for an Absorption Chiller under Different Working Conditions" Applied Sciences 7, no. 8: 797. https://doi.org/10.3390/app7080797
APA StyleWang, J., Shang, S., Li, X., Wang, B., Wu, W., & Shi, W. (2017). Dynamic Performance Analysis for an Absorption Chiller under Different Working Conditions. Applied Sciences, 7(8), 797. https://doi.org/10.3390/app7080797