1. Introduction
Various food products are sensitive to the external environment such as microbial attack [
1] and deterioration by atmospheric oxygen [
2]. These inevitably result in depreciation and a decrease in consumers’ preference [
3]. Antimicrobial packaging has been suggested by introducing nanoclays or antibacterial moiety in the film [
1,
4,
5]. Meanwhile, many researchers have studied gas barrier packaging or oxygen-scavenging materials enclosed in packaging. Svagan et al. reported that polylactide film with 40 bilayer coating of montmorillonite/chitosan reduced oxygen permeability by about 90–95% at 20–50% relative humidity (RH) [
6]. The preparation of carboxymethylated microfibrillated cellulose film was suggested to achieve low oxygen permeability at low RH by Aulin et al. [
7]. Another oxidation prevention strategy is to introduce oxygen-scavenging materials such as iron-based powder (iron oxide, ferrous carbonate and metallic platinum) [
8,
9], ascorbic acid with metal catalyst [
10], and an enzyme combination (glucose oxidase and catalase) into food packaging [
8]. Those oxygen scavengers are usually applied in the form of a sachet or film coating for the freshness of oxygen-sensitive food [
9,
11,
12].
However, there still remain several limitations in the aforementioned approaches. Although oxygen barrier film is effective to prevent O
2 access, only small damage in the film resulted in the failure of O
2 prevention. Oxygen scavengers like iron oxides requires relatively high humidity for their action [
8,
13]. Other scavengers like ascorbic acid or an enzyme combination are relatively expensive and not easy to deal with in a packaging process due to their environmental sensitivity (pH, salt condition, temperature and etc.) [
8,
10]. Therefore, we tried to prepare an alternative oxygen scavenger which has a low precursor price, controllable oxidation-reduction property, etc.
Green rust (GR) is a kind of layered nanomaterial consisting of Fe2+/Fe3+ mixed hydroxide layers and interlayer anion (Cl−, CO32− and SO42−) along with water. The structure is an analogue of layered double hydroxide, which is often referred to as an anionic nanoclay. The chemical composition of GR is similar to that of iron oxide; however, (1) its layered structure provides a larger surface for oxidation-reduction at the Fe2+/Fe3+ centers than iron oxide; and (2) the intrinsic water moiety along the hydroxide layer enabled a facilitated oxidation-reduction reaction. In fact, the Fe2+ in GR is quickly oxidized to Fe3+ in the presence of O2 to produce the goethite (FeOOH) phase. Thus, GR has high potential to capture O2 in food packaging as an oxygen-scavenging material. The only limitation of GR is its fast oxidation rate under the existence of O2, and thus GR itself can only be utilized as an O2 scavenger for short-term utility.
In order to overcome this problem and to control the oxidation-reduction reaction of GR, we tried to hybridize GR with a conducting polymer, poly(3,4-ethylenedioxythiophene) (PEDOT): poly(4-styrenesulfonate) (PSS), which have high electrical conductivity, transparency and thermal stability [
14,
15]. As the reduction potential of the conducting polymer would be slightly higher than Fe
2+, the polymer would retard the oxidation rate of GR. Furthermore, the electron donor-acceptor behavior between GR and the conducting polymer would control the oxidation rate of GR.
In this study, we prepared GR with general anion, SO42−, and conducting polymer (PEDOT:PSS), to compare the oxygen-scavenging property depending on the interlayer anion. In particular, GR with a SO42− interlayer anion (GR-SO4) was selected as reference sample of GR without a conducting polymer, as GR-SO4 is the most widely studied green rust in terms of structure and synthetic method. Through X-ray diffraction (XRD) patterns and X-ray absorption spectra (XAS) of both GRs, we confirmed the crystal and local structure of GR. In order to investigate the homogeneity in polymer distribution with GR, we measured the zeta-potential and scanning electron microscopic (SEM) images. Finally, we evaluated the time-dependent oxygen scavenging property of both GRs using O2 gas analyzer using ambient gas condition.
2. Materials and Methods
2.1. Materials
Reagent grade ferrous sulfate heptahydrate (FeSO4·7H2O), ferric sulfate hydrate (Fe2(SO4)3·9H2O), poly(3,4-ethylenedioxythiophene) (PEDOT) and poly(styrenesulfonate) (PSS) were purchased from Sigma-Aldrich Co. LLC (St. Louis, MO, USA). Sodium hydroxide pellets (NaOH) were obtained from Daejung Chemicals & Metals Co., Ltd. (Siheung, Korea). All the chemicals were used without further purification.
2.2. Preparation of Green Rust (GR)-SO4 and GR–PEDOT:PSS (GR–PEDOT)
For the preparation of GR-SO4, mixed divalent and trivalent iron solution (0.15 M of FeSO4∙7H2O and 0.05 M of Fe2(SO4)3∙9H2O) was titrated with 0.3 M of NaOH solution until pH reached ~6.9. This slurry was aged at room temperature for 24 h under N2 atmosphere. The conductive polymer-introduced GR (GR–PEDOT) was prepared under the same ferri/ferrous stoichiometry as GR-SO4 with an additional 5 mL of PEDOT:PSS solution (2.8 wt %). The alkaline solution was dropwisely added to the solution until pH ~6.9. The obtained product was stirred at room temperature for 24 h under N2 atmosphere. After 24 h, the final products were collected by centrifugation and lyophilized without washing process. Decarbonated water was used during synthesis process.
2.3. Characterization of GR-SO4 and GR–PEDOT
The crystal structure of GR-SO4 and GR-PEDOT was evaluated with a powder X-ray diffractometer (XRD; Bruker AXS D2 Phaser with LYNXEYE™ detector; Bruker AXS GmbH, Karlsruhe, Germany). The XRD patterns were measured with scanning range from 3° to 70° as following condition (1 mm air-scattering slit, a 0.1 mm equatorial slit, and time step increments of 0.02° and 0.5 s per step). The crystallite size was calculated using Scherrer’s equation (τ = Kλ/βcosθ, τ: crystallite size (Å), K: dimensionless shape factor (0.9), λ: X-ray wavelength (1.5406 Å), β: full-width at half-maximum, θ: Bragg angle). The surface charge of GR-SO4 and GR-PEDOT was investigated using an ELSZ-1000 (Otsuka, Kyoto, Japan). For zeta-potential measurement, each sample powder was dispersed in deionized water (1 mg/mL) and then the prepared suspension transferred to a quartz flow cell with platinum electrode. The particle morphology of GR-SO4 and GR-PEDOT was evaluated by scanning electron microscopy (FE-SEM; FEG Quanta250, FEI, USA). In order to obtain SEM images of samples, both powder samples were attached on carbon tape, and then sample surface was coated through Pt/Pd sputtering for 60 s. The SEM images were obtained under a 30 kV electron beam acceleration voltage with 10 mm working distance. The X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) spectra were obtained using X-ray absorption spectroscopy (XAS) in the 8C beam line at the Pohang Accelerator Laboratory (Pohang, Korea). To obtain XAS spectra, a powder sample was attached between kapton tapes, and then X-ray absorption was measured around Fe K-edge energy (7112 eV). The gained EXAFS spectra was analyzed with XAFSVIEW package in order to examine the local structure and coordination state around Fe.
2.4. Oxygen-Scavenging Activity
In order to evaluate oxygen scavenging activity of GR-SO4 and GR-PEDOT powder, both powders (0.6 g) were put in 60 mL volume airtight container which was previously filled with ambient air (~20 v% of O2). Then, the oxygen content of the internal container was measured using an O2 Headspace gas analyzer (CheckMate 3, MOCON Inc., Minneapolis, MN, USA) in a time-dependent manner (10, 20, 30, 40, 50 and 60 min).
3. Results
Figure 1 represents powder X-ray diffraction patterns of GR with SO
42− or with conducting polymer (PEDOT). The diffractogram of GR-SO
4, sulfate intercalated green rust showed well developed (00l) diffraction below 2θ of 30° and several small lattice peaks above 2θ of 30°. Similarly, the diffractogram of GR-PEDOT showed (00l) peaks and lattice peaks, respectively. The d-spacings of both GRs calculated by (003) peak was 0.84 and 1.08 nm, respectively, showing correspondence with the molecular dimension of SO
42− and PEDOT:PSS, respectively. It is notable that the GR-PEDOT showed a larger number of repeated (00l) peaks with higher intensity than GR-SO
4, which would be attributed to the different crystallinity of GR in the presence of the polymer. The crystallite sizes calculated by Scherrer’s equation [
16] utilizing (003) peaks were 7.36 and 10.5 nm for GR-SO
4 and GR-PEDOT, respectively. The (hkl) indexing followed the crystal phase of hydrotalcite (JCPDS NO.14-0191) as previously reported for green rust [
17].
The surface charge of both GRs were evaluated by measuring its zeta potential in deionized water (
Figure 2). The zeta potential of GR-SO
4 lay in the range of −10 mV to 30 mV, whereas that of GR-PEDOT was located between −40 mV and 0 mV. The full widths at half maximum of both samples were similarly narrow (~ 21 mV). The average zeta potential of GR-SO
4 and GR-PEDOT was 6.87 ± 0.44 mV and −19.8 ± 0.52 mV, respectively, clearly showing respective positive and negative surface charge.
Scanning electron microscopy for both samples was carried out in order to observe particle size, morphology and homogeneity in phase (
Figure 3). The image for GR-SO
4 showed relatively small particles with a size of 60 nm with some agglomeration. On the other hand, the particle of GR-PEDOT is larger and more plate-like than GR-SO
4. The average particle size of GR-PEDOT was 140 nm and it also showed some agglomerations. In the SEM image of GR-PEDOT, we could not observe any serious aggregation of particles or lumps of polymers, indicating the PEDOT:PSS was well hybridized with the GR lattice without phase segregation. Energy-dispersive spectroscopy (EDS) mapping images showed the location of GR framework was overlapped with those of counter-anions. The locations of S, Fe, O (Fe, O from GR; S, O from SO
42−) were well-overlapped in GR-SO
4; the locations of C, S, Fe, O (Fe, O from GR; C, S, O from PEDOT:PSS) were also well-overlapped in GR-PEDOT (
Figures S1 and S2). The photographic images of both GRs displayed in
Figure 3 showed fine powdery state with greenish brown or black color, indicating the existence of Fe
2+/Fe
3+ (for both samples) and PEDOT:PSS (in GR-PEDOT) in the powders.
Figure 4A showed XANES for GR-SO
4 and GR-PEDOT. Both spectra were similar in terms of peak shape and intensity. In particular, the pre-edge at 7114.04 eV and main edge at 7128.27 eV were commonly observed for both samples. The EXAFS in R-space was shown in
Figure 4B. There could be observed a strong first shell peak at 1.4 Å (non-phase-shift corrected) and a relatively small second shell peak at 2.6 Å (non-phase-shift corrected), attributed to Fe–O and Fe–Fe bonds, respectively. The peak positions and shapes corresponded well to the EXAFS spectra of previously reported green rust [
18,
19].
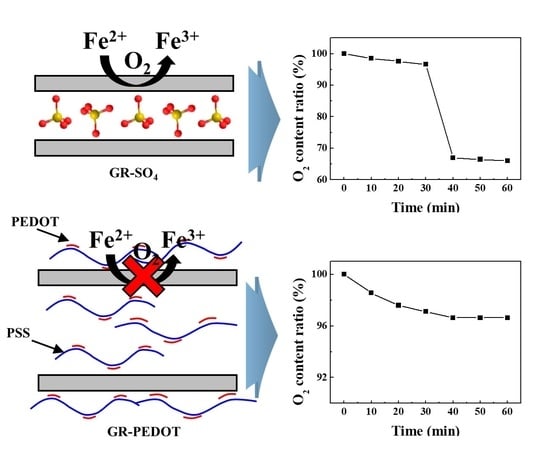
The oxygen-scavenging effect of GRs depending on different interlayer anions were evaluated in time-course (
Figure 5). The test was carried out under ambient composition of air where ~20% of oxygen existed. At the early stage until 30 min, both GR-SO
4 and GR-PEDOT slowly removed O
2 showing O
2 partial pressure reduction by 97%. From the 30-min time point, both GRs showed different behavior; the partial pressure of O
2 abruptly decreased to 66% for GR-SO
4. On the other hand, the partial pressure of O
2 in the GR-PEDOT-containing vessel continuously decreased following a logarithm function. The fitted curve for O
2 partial pressure in the GR-PEDOT vessel is as follows; P = 99.4 − 0.725ln(t − 6.93) (P: partial pressure of O
2, t: time in min).
4. Discussion
This research aims to prepare green rusts (GRs) with different interlayer anions and to evaluate their structure as well as O
2 scavenging property. As shown in
Scheme 1, the GR is composed of 2-dimensional layer and interlayer anions. The layers are composed of interconnected Fe(OH)
6 (either ferrous Fe
2+ or ferric Fe
3+) octahedrons in an edge-sharing manner. GRs are usually prepared with an interlayer ion of sulfate [
20] as shown in
Scheme 1a, while other small inorganic acids like carbonate and chloride are possible in the interlayer space [
21,
22]. Incorporation of organic moiety or polymers are rare in GR systems; however, recently, we reported that a combination of PSS and polypyrrole could be accommodated in a GR lattice increasing the crystallinity of the host GR [
23]. In the current study, we tried to intercalate a combination of conducting polymer (PEDOT:PSS) in order to exploit both the crystallinity increase and electron donation-acceptance property of conducting polymer.
As evidenced by X-ray diffractogram (
Figure 1), both GRs were successfully prepared with either SO
42− or PEDOT:PSS interlayer anions. The slightly higher peak intensity and larger crystallite size for GR-PEDOT than GR-SO
4 were attributed to the crystal growth role of the polymer as reported previously [
23]. The d-spacing of GR-SO
4 matched other literature well [
23], while the d-spacing value of GR-PEDOT, a newly synthesized phase, corresponded to the molecular dimension of PEDOT:PSS. We hypothesized that the benzene moiety of PSS aligned parallel to the GR layer, while the anionic center of sulfonate groups head to the GR layer for charge compensating (
Figure 1). As PSS acts as a dopant for PEDOT (
Scheme 1b), the PSS and PEDOT moiety would be overlapped in the interlayer space of GR. Taking into account the molecular dimension of PSS and PEDOT, the current d-spacing of 1.08 nm (0.60 nm for gallery height +0.48 nm for GR’s layer thickness) suggested that the GR layers were tightly holding the PEDOT:PSS moiety so that the electron transfer between PEDOT and GR layer could be feasible.
The positive zeta potential of GR-SO
4 (+6.87 mV) was attributed to the co-existence of Fe
2+ and Fe
3+ in the layer. The layer structure of GR could be understood from the brucite (Mg(OH)
2) layer. The substitution of Fe
3+ for Fe
2+ in a brucite-like layer, ferrous hydroxide (Fe(II)(OH)
2), generated an excessive plus charge [
24] resulting in positive zeta potential. It was noteworthy that GR-PEDOT had a negative charge surface about −19.8 mV. According to the previous research of other groups, the surface charge of PEDOT:PSS was negative (around −80~−89 mv) at various PSS/PEDOT ratios due to coverage by the negatively charged PSS-rich layer [
25,
26]. As the surface of GR-PEDOT would be homogenously covered with PEDOT:PSS, as the polymer does in the interlayer space, the zeta potential inevitably shifted to the negative region. We excluded the possibility that the PEDOT:PSS was simply mixed with GR particles. If that case occurred, the zeta potential of GR-PEDOT would be located in a more negative region (lower than −19.8 mV) or two peaks, one for PEDOT:PSS and the other for GR, would be observed. The slight negative charge of GR-PEDOT compared with PEDOT:PSS indicated homogeneous hybridization between GR and PEDOT:PSS, hopefully through intercalation and surface coating. The SEM and EDS mapping images also convinced us that homogenous hybridization between PEDOT:PSS and GR was successful in GR-PEDOT (
Figure 3 and
Figure S2). We could clearly observe the grain of particles in SEM images of both GR-SO
4 and GR-PEDOT. Simple mixing between polymer and GR would result in two different morphologies in the microscopic image.
The photographs for powder revealed that both Fe
2+ and Fe
3+ co-exist in the synthesized GR samples (
Figure 3). As the oxidation of Fe
2+ to Fe
3+ is spontaneous in terms of thermodynamics, goethite (Fe(III)OOH) impurity was often obtained during GR synthesis. The greenish color for both samples indicated that oxidation of Fe
2+ was efficiently prohibited and the GR phase was obtained well. This result corresponded well to the XRD pattern (
Figure 1) with only GR (hydrotalcite) peaks observed.
From XAS spectroscopy, we could confirm that the local structure of GR was not significantly affected by the type of intercalated molecules. The XANES spectra (
Figure 4a) of both GRs showed same main edge (1s → 4p transition) absorption [
27], and thus the average oxidation state of Fe was almost similar to each other. There existed a pre-edge for both GRs, implying the potential Jahn–Teller distortion around Fe. Those kinds of pre-edge could be interpreted as symmetry that allowed 1s → 3d electron transition [
18]. Although the Fe(OH)
6 in GR is considered to have octahedral geometry, the real symmetry is rather assigned to D
3d due to the packing of octahedrons [
28]. In terms of the edge positions (pre-edge and main edge) as well as the shape of the white line, both GRs would have same local symmetry around Fe. The R-space EXAFS patterns were also similar for both GR-SO
4 and GR-PEDOT. This result indicated that the inter-atomic distances of both Fe–O and Fe–Fe were not affected by different interlayer molecules. It is, therefore, concluded that both GRs had the same structure of layer framework and different interlayer and/or surface chemistry.
The difference in interlayer anions is thought to influence the oxidation of Fe2+ in GR. The partial O2 pressure gradually decreased for both GR-SO4 and GR-PEDOT until 30 min. After then, the pattern became different; GR-PEDOT kept gradual decreasing while GR-SO4 decreased O2 partial pressure abruptly. At time point 60 min, the difference in O2 partial pressure between GR-SO4 and the GR-PEDOT treated group was ~30%. This result suggested that the oxidation of GR was retarded in the presence of the conducting polymer, PEDOT:PSS. Applying a time vs. O2 pressure equation, p = 99.4 − 0.725ln(t − 6.93) (p: partial pressure of O2, t: time in min), we could extrapolate that O2 scavenging would continuously occur by GR-PEDOT for a prolonged time.
In general, the oxidation process of Fe
2+ (−0.506 V vs. saturated calomel electrode (SCE)) is thermodynamically favored combined with the reduction of oxygen (0.57 V vs. SCE) so that the green rust could be easily transformed to goethite (Fe(III)OOH) under ambient air as we reported in the previous literature [
22]. However, the oxidation potential of PEDOT:PSS is relatively higher than that of Fe
2+ [
29,
30], and thus the oxidation of PEDOT by O
2 is less feasible than oxidation of Fe
2+. The action of PEDOT on the GR surface could be (i) protecting GR from oxidation, and (ii) retarding oxidation by atmospheric O
2 through adjusted reduction potential. Therefore, it could be suggested that
Figure 5a showed the oxygen-scavenging profile by the transformation of green rust to goethite without PEDOT:PSS but PEDOT:PSS could retard the oxidation as shown in
Figure 5b.