Novel Polymer–Silica Composite-Based Bifunctional Catalysts for Hydrodeoxygenation of 4-(2-Furyl)-3-Buten-2-One as Model Substance for Furfural–Acetone Aldol Condensation Products
Abstract
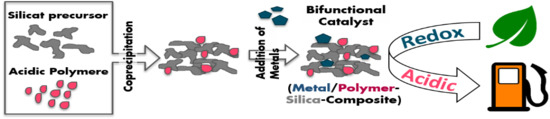
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals Used
2.2. Catalyst Preparation
2.3. Catalyst Characterization
2.4. Catalytic Investiagtions
3. Results
3.1. Catalyst Properties
3.2. Catalytic Investigations
3.2.1. Influence of Polymer Content/Acidic Properties
3.2.2. Influence of Platinum Content/Redox Properties
3.2.3. Influence of the Noble Metal
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chheda, J.N.; Dumesic, J.A. An overview of dehydration, aldol-condensation and hydrogenation processes for production of liquid alkanes from biomass-derived carbohydrates. Catal. Today 2007, 123, 59–70. [Google Scholar] [CrossRef]
- Stocker, M. Biofuels and biomass-to-liquid fuels in the biorefinery: Catalytic conversion of lignocellulosic biomass using porous materials. Angew. Chem. Int. Ed. 2008, 47, 9200–9211. [Google Scholar] [CrossRef] [PubMed]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Subrahmanyam, A.V.; Olcay, H.; Qi, W.; van Walsum, G.P.; Pendse, H.; Huber, G.W. Production of jet and diesel fuel range alkanes from waste hemicellulose-derived aqueous solutions. Green Chem. 2010, 12, 1933. [Google Scholar] [CrossRef]
- Lange, J.-P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural—A promising platform for lignocellulosic biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Faba, L.; Diaz, E.; Ordonez, S. One-pot aldol condensation and hydrodeoxygenation of biomass-derived carbonyl compounds for biodiesel synthesis. ChemSusChem 2014, 7, 2816–2820. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Kubička, D.; Čejka, J. Toward understanding of the role of Lewis acidity in aldol condensation of acetone and furfural using MOF and zeolite catalysts. Catal. Today 2015, 243, 158–162. [Google Scholar] [CrossRef]
- Kubička, D.; Kikhtyanin, O. Opportunities for zeolites in biomass upgrading—Lessons from the refining and petrochemical industry. Catal. Today 2015, 243, 10–22. [Google Scholar] [CrossRef]
- Barrett, C.J.; Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Single-reactor process for sequential aldol-condensation and hydrogenation of biomass-derived compounds in water. Appl. Catal. B Environ. 2006, 66, 111–118. [Google Scholar] [CrossRef]
- Faba, L.; Díaz, E.; Ordóñez, S. Hydrodeoxygenation of acetone–furfural condensation adducts over alumina-supported noble metal catalysts. Appl. Catal. B Environ. 2014, 160, 436–444. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Bulanek, R.; Frolich, K.; Cejka, J.; Kubicka, D. Aldol condensation of furfural with acetone over ion-exchanged and impregnated potassium BEA zeolites. J. Mol. Catal. A Chem. 2016, 424, 358–368. [Google Scholar] [CrossRef]
- Van der Wal, H.; Sperber, B.L.H.M.; Houweling-Tan, B.; Bakker, R.R.C.; Brandenburg, W.; Lopez-Contreras, A.M. Production of acetone, butanol, and ethanol from biomass of the green seaweed Ulva lactuca. Bioresour. Technol. 2013, 128, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.; Shi, D.; Sooknoi, T.; Resasco, D.E. Aqueous-phase ketonization of acetic acid over Ru/TiO2/carbon catalysts. J. Catal. 2012, 295, 169–178. [Google Scholar] [CrossRef]
- Chatterjee, M.; Matsushima, K.; Ikushima, Y.; Sato, M.; Yokoyama, T.; Kawanami, H.; Suzuki, T. Production of linear alkane via hydrogenative ring opening of a furfural-derived compound in supercritical carbon dioxide. Green Chem. 2010, 12, 779. [Google Scholar] [CrossRef]
- Ramos, R.; Tisler, Z.; Kikhtyanin, O.; Kubicka, D. Solvent effects in hydrodeoxygenation of furfural-acetone aldol condensation products over Pt/TiO2 catalyst. Appl. Catal. A Gen. 2017, 530, 174–183. [Google Scholar] [CrossRef]
- Luska, K.L.; Julis, J.; Stavitski, E.; Zakharov, D.N.; Adams, A.; Leitner, W. Bifunctional nanoparticle–SILP catalysts (NPs@SILP) for the selective deoxygenation of biomass substrates. Chem. Sci. 2014, 5, 4895–4905. [Google Scholar] [CrossRef]
- Xia, Q.; Xia, Y.; Xi, J.; Liu, X.; Wang, Y. Energy-efficient production of 1-octanol from biomass-derived furfural-acetone in water. Green Chem. 2015, 17, 4411–4417. [Google Scholar] [CrossRef]
- Harmer, M.A.; Farneth, W.E.; Sun, Q. High Surface Area Nafion † Resin/Silica Nanocomposites: A New Class of Solid Acid Catalyst. J. Am. Chem. Soc. 1996, 118, 7708–7715. [Google Scholar] [CrossRef]
- Gallo, J.M.R.; Alamillo, R.; Dumesic, J.A. Acid-functionalized mesoporous carbons for the continuous production of 5-hydroxymethylfurfural. J. Mol. Catal. A Chem. 2016, 422, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Ozbay, N.; Oktar, N.; Dogu, G.; Dogu, T. Activity Comparison of Different Solid Acid Catalysts in Etherification of Glycerol with tert-Butyl Alcohol in Flow and Batch Reactors. Top. Catal. 2013, 56, 1790–1803. [Google Scholar] [CrossRef]
- Liu, Y.; Lotero, E.; Goodwinjr, J.G. Effect of carbon chain length on esterification of carboxylic acids with methanol using acid catalysis. J. Catal. 2006, 243, 221–228. [Google Scholar] [CrossRef]
- Meylemans, H.A.; Quintana, R.L.; Rex, M.L.; Harvey, B.G. Low-temperature, solvent-free dehydration of cineoles with heterogeneous acid catalysts for the production of high-density biofuels. J. Chem. Technol. Biotechnol. 2014, 89, 957–962. [Google Scholar] [CrossRef]
- Wang, H.; Xu, B.-Q. Catalytic performance of Nafion/SiO2 nanocomposites for the synthesis of α-tocopherol. Appl. Catal. A Gen. 2004, 275, 247–255. [Google Scholar] [CrossRef]
- Harmer, M.A.; Sun, Q.; Vega, A.J.; Farneth, W.E.; Heidekum, A.; Hoelderich, W.F. Nafion resin–silica nanocomposite solid acid catalysts. Microstructure–processing–property correlations. Green Chem. 2000, 2, 7–14. [Google Scholar] [CrossRef]
- Schuster, H.; Hoelderich, W.F. The acylation of 2-methoxynaphthalene with acetic anhydride over Nafion/silica composites and BEA zeolites containing Lewis acid sites. Appl. Catal. A Gen. 2008, 350, 1–5. [Google Scholar] [CrossRef]
- Harmer, M.A.; Farneth, W.E.; Sun, Q. Towards the Sulfuric Acid of Solids. Adv. Mater. 1998, 10, 1255–1257. [Google Scholar] [CrossRef]
- Laufer, W.; Niederer, J.P.M.; Hoelderich, W.F. New Direct Hydroxylation of Benzene with Oxygen in the Presence of Hydrogen over Bifunctional Palladium/Platinum Catalysts. Adv. Synth. Catal. 2002, 344, 1084–1089. [Google Scholar] [CrossRef]
- Laufer, W.; Hoelderich, W.F. New direct hydroxylation of benzene with oxygen in the presence of hydrogen over bifunctional ion-exchange resins. Chem. Commun. 2002, 1684–1685. [Google Scholar] [CrossRef]
- Ramos, R.; Tišler, Z.; Kikhtyanin, O.; Kubička, D. Towards understanding the hydrodeoxygenation pathways of furfural–acetone aldol condensation products over supported Pt catalysts. Catal. Sci. Technol. 2016, 6, 1829–1841. [Google Scholar] [CrossRef]
- Xia, Q.-N.; Cuan, Q.; Liu, X.-H.; Gong, X.-Q.; Lu, G.-Z.; Wang, Y.-Q. Pd/NbOPO4 multifunctional catalyst for the direct production of liquid alkanes from aldol adducts of furans. Angew. Chem. Int. Ed. 2014, 53, 9755–9760. [Google Scholar] [CrossRef]
- Liu, Y.; Lotero, E.; Goodwinjr, J.G. A comparison of the esterification of acetic acid with methanol using heterogeneous versus homogeneous acid catalysis. J. Catal. 2006, 242, 278–286. [Google Scholar] [CrossRef]
- Xu, W.; Xia, Q.; Zhang, Y.; Guo, Y.; Wang, Y.; Lu, G. Effective production of octane from biomass derivatives under mild conditions. ChemSusChem 2011, 4, 1758–1761. [Google Scholar] [CrossRef] [PubMed]
- Waidmann, C.R.; Pierpont, A.W.; Batista, E.R.; Gordon, J.C.; Martin, R.L.; “Pete” Silks, L.A.; West, R.M.; Wu, R. Functional group dependence of the acid catalyzed ring opening of biomass derived furan rings: An experimental and theoretical study. Catal. Sci. Technol. 2013, 3, 106–115. [Google Scholar] [CrossRef]
- Shao, Y.; Xia, Q.; Liu, X.; Lu, G.; Wang, Y. Pd/Nb2O5/SiO2 catalyst for the direct hydrodeoxygenation of biomass-related compounds to liquid alkanes under mild conditions. ChemSusChem 2015, 8, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Kikhtyanin, O.; Kelbichová, V.; Vitvarová, D.; Kubů, M.; Kubička, D. Aldol condensation of furfural and acetone on zeolites. Catal. Today 2014, 227, 154–162. [Google Scholar] [CrossRef]
- Bedia, J.; Rosas, J.M.; Vera, D.; Rodríguez-Mirasol, J.; Cordero, T. Isopropanol decomposition on carbon based acid and basic catalysts. Catal. Today 2010, 158, 89–96. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Wang, S. Hydrodeoxygenation of bio-oil over Pt-based supported catalysts: Importance of mesopores and acidity of the support to compounds with different oxygen contents. RSC Adv. 2013, 3, 12635. [Google Scholar] [CrossRef]
- Michel, C.; Gallezot, P. Why Is Ruthenium an Efficient Catalyst for the Aqueous-Phase Hydrogenation of Biosourced Carbonyl Compounds? ACS Catal. 2015, 5, 4130–4132. [Google Scholar] [CrossRef]
- Pritchard, J.; Filonenko, G.A.; van Putten, R.; Hensen, E.J.M.; Pidko, E.A. Heterogeneous and homogeneous catalysis for the hydrogenation of carboxylic acid derivatives: History, advances and future directions. Chem. Soc. Rev. 2015, 44, 3808–3833. [Google Scholar] [CrossRef]






| Catalyst | ABET/(m2 g−1) | Vpore/(cm3 g−1) | dpore/nm | Polymer Content/wt.% | Metal Content/wt.% |
|---|---|---|---|---|---|
| (1)Pt(0)PSC | 182 | 0.40 | 9.1 | 0.0 | 0.91 |
| (1)Pt(6.5)PSC | 168 | 0.38 | 9.0 | 6.3 | 0.95 |
| (13)PSC | 134 | 0.30 | 9.2 | 13.1 | n.d. |
| (0.5)Pt(13)PSC | 125 | 0.29 | 9.0 | 12.7 | 0.43 |
| (1)Pt(13)PSC | 131 | 0.35 | 8.9 | 12.6 | 0.92 |
| (2)Pt(13)PSC | 130 | 0.33 | 9.1 | 12.8 | 1.83 |
| (1)Pt(26)PSC | 105 | 0.24 | 9.5 | 25.1 | 0.95 |
| (1)Pd(13)PSC | 133 | 0.33 | 8.9 | 12.8 | 0.92 |
| (1)Ru(13)PSC | 115 | 0.34 | 9.0 | 12.5 | 0.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goepel, M.; Ramos, R.; Gläser, R.; Kubička, D. Novel Polymer–Silica Composite-Based Bifunctional Catalysts for Hydrodeoxygenation of 4-(2-Furyl)-3-Buten-2-One as Model Substance for Furfural–Acetone Aldol Condensation Products. Appl. Sci. 2019, 9, 2438. https://doi.org/10.3390/app9122438
Goepel M, Ramos R, Gläser R, Kubička D. Novel Polymer–Silica Composite-Based Bifunctional Catalysts for Hydrodeoxygenation of 4-(2-Furyl)-3-Buten-2-One as Model Substance for Furfural–Acetone Aldol Condensation Products. Applied Sciences. 2019; 9(12):2438. https://doi.org/10.3390/app9122438
Chicago/Turabian StyleGoepel, Michael, Ruben Ramos, Roger Gläser, and David Kubička. 2019. "Novel Polymer–Silica Composite-Based Bifunctional Catalysts for Hydrodeoxygenation of 4-(2-Furyl)-3-Buten-2-One as Model Substance for Furfural–Acetone Aldol Condensation Products" Applied Sciences 9, no. 12: 2438. https://doi.org/10.3390/app9122438
APA StyleGoepel, M., Ramos, R., Gläser, R., & Kubička, D. (2019). Novel Polymer–Silica Composite-Based Bifunctional Catalysts for Hydrodeoxygenation of 4-(2-Furyl)-3-Buten-2-One as Model Substance for Furfural–Acetone Aldol Condensation Products. Applied Sciences, 9(12), 2438. https://doi.org/10.3390/app9122438