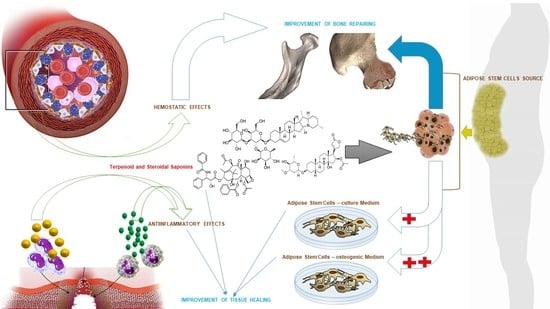
The Synergic Effect of Terpenoid and Steroidal Saponins Can Improve Bone Healing, by Promoting the Osteogenic Commitment of Adipose Mesenchymal Stem Cells: An In Vitro Study
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Mesenchymal Stem Cells from Adipose Tissue (ADSCs)
2.2. Cell Culture
2.3. Methyl Thiazolyl-Tetrazolium (MTT) Assay
2.4. Real-Time PCR Assay
2.5. Alkaline Phosphatase Assay
2.6. Alizarin Red S Staining Assay
3. Results
3.1. ADSCs Viability with Different YB Concentrations
3.2. Impact of YB on Osteogenic Gene Expression
3.3. Impact of YB on Alkaline Phosphatase Activity
3.4. Impact of YB on Alizarin Red S Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Foulke, B.A.; Kendal, A.R.; Murray, D.W.; Pandit, H. Fracture healing in the elderly: A review. Maturitas 2016, 92, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.; Bikle, D.D. Osteogenic Differentiation of Periosteal Cells During Fracture Healing. J. Cell. Physiol. 2017, 232, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.N.; Hankenson, K.D. Mesenchymal Stem Cells in Bone Regeneration. Adv. Wound Care 2013, 2, 306–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Janghorbani, M.; Van Dam, R.M.; Willett, W.C.; Hu, F.B. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am. J. Epidemiol. 2007, 166, 495–505. [Google Scholar] [CrossRef]
- Folk, J.W.; Starr, A.J.; Early, J.S. Early wound complications of operative treatment of calcaneus fractures: Analysis of 190 fractures. J. Orthop. Trauma 1999, 13, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Loder, R.T. The influence of diabetes mellitus on the healing of closed fractures. Clin. Orthop. Relat. Res. 1988, 232, 210–216. [Google Scholar] [CrossRef]
- Retzepi, M.; Donos, N. The effect of diabetes mellitus on osseous healing. Clin. Oral Implants Res. 2010, 21, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Luyten, F.P.; Van der Schueren, B.; Kerckhofs, G.; Vandamme, K. The Impact of Type 2 Diabetes on Bone Fracture Healing. Front. Endocrinol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Ren, X.; Wang, X.; Shi, X.; Wang, X.; Ding, Z.; Gao, P.; Xu, G. Therapeutic effect of Yunnan Baiyao on rheumatoid arthritis was partially due to regulating arachidonic acid metabolism in osteoblasts. J. Pharm. Biomed. Anal. 2012, 59, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Wang, L.; Chen, X.Q.; Deng, X.T.; Cao, Y.; Wang, Q. Simultaneous quantification of both triterpenoid and steroidal saponins in various Yunnan Baiyao preparations using HPLC–UV and HPLC–MS. J. Sep. Sci. 2008, 31, 3834–3846. [Google Scholar] [CrossRef] [PubMed]
- Polesuk, J.; Amodeo, J.M.; Ma, T.S. Microchemical investigation of medicinal plants. X. Microchim. Acta 1973, 61, 507–517. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Y.; Wang, Q.; Zhang, T.; Song, Y. Detection of saponins in extracts from the rhizomes of Paris species and prepared Chinese medicines by high performance liquid chromatography-electrospray ionization mass spectrometry. Planta Med. 2006, 72, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Wirth, K.A.; Kow, K.; Salute, M.E.; Bacon, N.J.; Milner, R.J. In vitro effects of Yunnan Baiyao on canine hemangiosarcoma cell lines. Vet. Comp. Oncol. 2016, 14, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Wang, S.F.; Hu, Z.M. The Effects of Yunnan Baiyao on Bone Defects Healing and Guided Bone Regeneration. Chin. J. Orthop. 2002, 8. [Google Scholar]
- Liang, N.; Zou, Y.; Zhangh, H. Comparison of curative effects between Yunnan Baiyao and iodoform applied in rats after cleft palate operation. J. Jilin Univ. Med. Ed. 2013, 39, 893–897. [Google Scholar]
- Ou, Z.; Cheng, Q.; Chen, Y.; Chen, T.; Rong, X.; Long, F.; Zhang, X.; Liang, Q.; Feng, Z. Chemical characterization of wound ointment (WO) and its effects on fracture repair: A rabbit model. Chin. Med. 2017, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, X.; Zhang, W.; Zhao, J.Z.; Dong, H. Effect of Yunnan Baiyao capsules on the socket healing of impacted mandibular third molar extraction. Chin. J. Stomatol. 2012, 47, 199–202. [Google Scholar] [CrossRef]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 85, 747–754. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, J.M.; Im, G.I. Electroporation-mediated transfer of Runx2 and Osterix genes to enhance osteogenesis of adipose stem cells. Biomaterials 2011, 32, 760–768. [Google Scholar] [CrossRef]
- Marom, R.; Shur, I.; Solomon, R.; Benayahu, D. Characterization of adhesion and differentiation markers of osteogenic marrow stromal cells. J. Cell. Physiol. 2005, 202, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Noh, W.C.; Park, J.W.; Lee, J.M.; Suh, J.Y. Gene expression pattern during osteogenic differentiation of human periodontal ligament cells in vitro. J. Periodontal Implant Sci. 2011, 41, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Desai, H.V.; Voruganti, I.S.; Jayasuriya, C.; Chen, Q.; Darling, E.M. Live-cell, temporal gene expression analysis of osteogenic differentiation in adipose-derived stem cells. Tissue Eng. A 2013, 19, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.T.; Nah, W.K.; Gupta, A.; Hutmacher, D.W.; Woodruff, M.A. The osteogenic differentiation of adipose tissue-derived precursor cells in a 3D scaffold/matrix environment. Curr. Drug Discov. Technol. 2008, 5, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Nishimoto, S.K. Radioimmunoassay for the vitamin K-dependent protein of bone and its discovery in plasma. Proc. Natl. Acad. Sci. USA 1980, 77, 2234–2238. [Google Scholar] [CrossRef] [PubMed]
- Sila-Asna, M.; Bunyaratvej, A.; Maeda, S.; Kitaguchi, H.; Bunyaratavej, N. Osteoblast Differentiation and Bone Formation Gene Expression in Strontium-inducing Bone Marrow Mesenchymal Stem Cell. Kobe J. Med. Sci. 2007, 53, 25–35. [Google Scholar] [PubMed]
- Lu, H.; Kraut, D.; Gerstenfeld, L.C.; Graves, D.T. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology 2013, 144, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Roy, B. Biomolecular basis of the role of diabetes mellitus in osteoporosis and bone fractures. World J. Diabetes 2015, 4, 101–113. [Google Scholar] [CrossRef]
- Brown, M.L.; Yukata, K.; Farnsworth, C.W.; Chen, D.G.; Awad, H.; Hilton, M.J.; O’Keefe, R.J.; Xing, L.; Mooney, R.A.; Zuscik, M.J. Delayed fracture healing and increased callus adiposity in a C57BL/6J murine model of obesity-associated type 2 diabetes mellitus. PLoS ONE 2014, 9, e99656. [Google Scholar] [CrossRef]
- Sarkar, P.D.; Choudhury, A.B. Relationships between serum osteocalcin levels versus blood glucose, insulin resistance and markers of systemic inflammation in central Indian type 2 diabetic patients. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1631–1635. [Google Scholar]
- Olimpio, R.M.C.; De Oliveira, M.; De Sibio, M.T.; Moretto, F.C.F.; Deprá, I.C.; Mathias, L.S.; Gonçalves, B.M.; Rodrigues, B.M.; Tilli, H.P.; Coscrato, V.E.; et al. Cell viability assessed in a reproducible model of human osteoblasts derived from human adipose-derived stem cells. PLoS ONE 2018, 13, e0194847. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Wang, Y.; Wu, J.; Zhou, Z.; Xu, T.; Jin, L.; Yu, Y.; Li, Z.; Gobin, R.; Xue, C.; et al. Yunnan Baiyao Conditioned Medium Promotes the Odonto/Osteogenic Capacity of Stem Cells from Apical Papilla via Nuclear Factor Kappa B Signaling Pathway. BioMed Res. Int. 2019, 2019, 9327386. [Google Scholar] [CrossRef] [PubMed]
- Coen, K.; Flannagan, R.S.; Baron, S.; Carraro-Lacroix, L.R.; Wang, D.; Vermeire, W.; Michiels, C.; Munck, S.; Baert, V.; Sugita, S.; et al. Lysosomal calcium homeostasis defects, not proton pump defects, cause endo-lysosomaldysfunction in PSEN-deficient cells. J. Cell Biol. 2012, 198, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Tai, V.; Leung, W.; Grey, A.; Reid, I.R.; Bolland, M.J. Calcium intake and bone mineral density: Systematic review and meta-analysis. BMJ 2015, 351, 4183. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; Gardin, C.; Bressan, E.; Calvo-Guirado, J.L.; Isola, M.; Piattelli, A.; Zavan, B. Ionized Ti Surfaces Increase Cell Adhesion Properties of Mesenchymal Stem Cells. J. Biomater. Tissue Eng. 2015, 5, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Paduano, S.; Uomo, R.; Amato, M.; Riccitiello, F.; Simeone, M.; Valletta, R. Cyst-like periapical lesion healing in an orthodontic patient: A case report with five-year follow-up. G. Ital. Endod. 2013, 27, 95–104. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; Alom, N.; Amer, M.; White, L.J.; Shakesheff, K.M.; Tatullo, M. Decellularized bone extracellular matrix and human dental pulp stem cells as a construct for bone regeneration. J. Biomater. Sci. Polym. Ed. 2017, 28, 730–748. [Google Scholar] [CrossRef] [Green Version]
- Di Vito, A.; Giudice, A.; Chiarella, E.; Malara, N.; Bennardo, F.; Fortunato, L. In vitro long-term expansion and high osteogenic potential of periodontal ligament stem cells: More than a mirage. Cell Transplant. 2019, 28, 129–139. [Google Scholar] [CrossRef]
- Currò, M.; Matarese, G.; Isola, G.; Caccamo, D.; Ventura, V.P.; Cornelius, C.; Lentini, M.; Cordasco, G.; Ientile, R. Differential expression of transglutaminase genes in patients with chronic periodontitis. Oral Dis. 2014, 20, 616–623. [Google Scholar] [CrossRef]
- Matarese, G.; Ramaglia, L.; Fiorillo, L.; Cervino, G.; Lauritano, F.; Isola, G. Implantology and Periodontal Disease: The Panacea to Problem Solving? Open Dent. J. 2017, 11, 460–465. [Google Scholar] [CrossRef]
- Isola, G.; Ramaglia, L.; Cordasco, G.; Lucchese, A.; Fiorillo, L.; Matarese, G. The effect of a functional appliance in the management of temporomandibular joint disorders in patients with juvenile idiopathic arthritis. Minerva Stomatol. 2017, 66, 1–8. [Google Scholar] [PubMed]
- Marrelli, M.; Tatullo, M.; Dipalma, G.; Inchingolo, F. Oral infection by staphylococcus aureus in patients affected by white sponge nevus: A description of two cases occurred in the same family. Int. J. Med. Sci. 2012, 9, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Figliuzzi, M.M.; Giudice, A.; Pileggi, S.; Pacifico, D.; Marrelli, M.; Tatullo, M.; Fortunato, L. Implant-prosthetic rehabilitation in bilateral agenesis of maxillary lateral incisors with a mini split crest. Case Rep. Dent. 2016, 2016, 3591321. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Gentile, M.; Inchingolo, A.M.; Dipalma, G. Non-syndromic multiple supernumerary teeth in a family unit with a normal karyotype: Case report. Int. J. Med. Sci. 2010, 7, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, M.; Amantea, M.; Paduano, F.; Santacroce, L.; Gentile, S.; Scacco, S. Bioimpedance Detection of Oral Lichen Planus Used as Preneoplastic Model. J. Cancer 2015, 6, 976–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Inchingolo, A.M.; Dipalma, G. Non-Hodgkin lymphoma affecting the tongue: Unusual intra-oral location. Head Neck Oncol. 2011, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Bennardo, F.; Barone, S.; Antonelli, A.; Figliuzzi, M.M.; Fortunato, L. Can autofluorescence guide surgeons in the treatment of medication-related osteonecrosis of the jaw? A prospective feasibility study. J. Oral Maxillofac. Surg. 2018, 76, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Marrelli, M.; Scacco, S.; Lorusso, M.; Doria, S.; Sabatini, R.; Auteri, P.; Cagiano, R.; Inchingolo, F. Relationship between oxidative stress and “burning mouth syndrome” in female patients: A scientific hypothesis. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1218–1221. [Google Scholar] [PubMed]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Palladino, A.; Inchingolo, A.M.; Dipalma, G. Oral piercing and oral diseases: A short time retrospective study. Int. J. Med. Sci. 2011, 8, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Paduano, F.; Marrelli, M.; Amantea, M.; Rengo, C.; Rengo, S.; Goldberg, M.; Spagnuolo, G.; Tatullo, M. Adipose Tissue as a Strategic Source of Mesenchymal Stem Cells in Bone Regeneration: A Topical Review on the Most Promising Craniomaxillofacial Applications. Int. J. Mol. Sci. 2017, 18, 2140. [Google Scholar] [CrossRef]





| Gene | Sequences (5′–3′) | Amplicon (pb) |
|---|---|---|
| RUNX2 | AGCCTTACCAAACAACACAACAG | 175 |
| CCATATGTCCTCTCAGCTCAGC | ||
| ALPL | GGCTTCTTCTTGCTGGTGGA | 181 |
| CAAATGTGAAGACGTGGGAATGG | ||
| OC | GCAGCGAGGTAGTGAAGAGAC | 193 |
| AGCAGAGCGACACCCTA | ||
| TFRC | TGTTTGTCATAGGGCAGTTGGAA | 222 |
| ACACCCGAACCAGGAATCTC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellin, G.; Gardin, C.; Ferroni, L.; Ghensi, P.; Zavan, B.; Tatullo, M. The Synergic Effect of Terpenoid and Steroidal Saponins Can Improve Bone Healing, by Promoting the Osteogenic Commitment of Adipose Mesenchymal Stem Cells: An In Vitro Study. Appl. Sci. 2019, 9, 3426. https://doi.org/10.3390/app9163426
Bellin G, Gardin C, Ferroni L, Ghensi P, Zavan B, Tatullo M. The Synergic Effect of Terpenoid and Steroidal Saponins Can Improve Bone Healing, by Promoting the Osteogenic Commitment of Adipose Mesenchymal Stem Cells: An In Vitro Study. Applied Sciences. 2019; 9(16):3426. https://doi.org/10.3390/app9163426
Chicago/Turabian StyleBellin, Gloria, Chiara Gardin, Letizia Ferroni, Paolo Ghensi, Barbara Zavan, and Marco Tatullo. 2019. "The Synergic Effect of Terpenoid and Steroidal Saponins Can Improve Bone Healing, by Promoting the Osteogenic Commitment of Adipose Mesenchymal Stem Cells: An In Vitro Study" Applied Sciences 9, no. 16: 3426. https://doi.org/10.3390/app9163426
APA StyleBellin, G., Gardin, C., Ferroni, L., Ghensi, P., Zavan, B., & Tatullo, M. (2019). The Synergic Effect of Terpenoid and Steroidal Saponins Can Improve Bone Healing, by Promoting the Osteogenic Commitment of Adipose Mesenchymal Stem Cells: An In Vitro Study. Applied Sciences, 9(16), 3426. https://doi.org/10.3390/app9163426