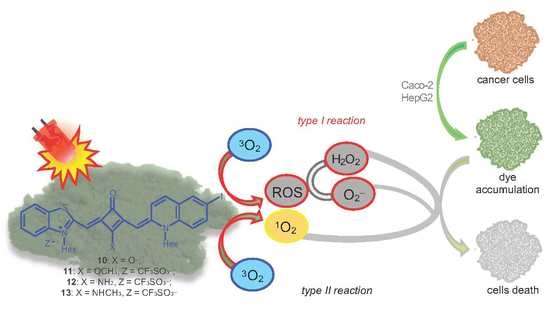
Photophysicochemical Properties and In Vitro Phototherapeutic Effects of Iodoquinoline- and Benzothiazole-Derived Unsymmetrical Squaraine Cyanine Dyes
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. Synthesis of 2-[(1-hexyl-6-iodoquinolin-2(1H)-ylidene)methyl]-4-[(3-hexylbenzothiazol-3-ium-2-yl)methylene)- 3-oxocyclobut-1-en-1-olate (10)
2.1.2. Synthesis of 3-hexyl-2-[(3-[(1-hexyl-6-iodoquinolin-2(1H)-ylidene)methyl]-2-methoxy-4-oxocyclobut-2-en-1-ylidene)methyl]benzothiazol-3-ium trifluoromethanesulfonate (11)
2.1.3. Synthesis of 2-[(2-amino-3-[(1-hexyl-6-iodoquinolin-2(1H)-ylidene)methyl]-4-oxocyclobut-2-en-1-ylidene)methyl]-3-hexylbenzothiazol-3-ium trifluoromethanesulfonate (12)
2.1.4. Synthesis of 3-hexyl-2-[(3-[(1-hexyl-6-iodoquinolin-2(1H)-ylidene)methyl]-2-methylamino-4-oxocyclobut-2-en-1-ylidene)methyl]benzothiazol-3-ium trifluoromethanesulfonate (13)
2.2. Singlet Oxygen Formation Quantum Yields Measurement
2.3. Photostability Monitoring
2.4. In Vitro Photobiological Evaluation
Data Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Photophysical Properties
3.3. Photodegradation Evaluation
3.4. Photocytotoxicity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cohen, S.; Lacher, J.R.; Park, J.D. Diketocyclobutenediol. J. Am. Chem. Soc. 1959, 81, 3480. [Google Scholar] [CrossRef]
- Hollander, F.J.; Semmingsen, D.; Koetzle, T.F. The molecular and crystal structure of squaric acid (3,4-dihydroxy-3-cyclobutene-1,2-dione) at 121 °C: A neutron diffraction study. J. Chem. Phys. 1977, 67, 4825–4831. [Google Scholar] [CrossRef]
- Ito, M.; West, R. New Aromatic Anions. IV. Vibrational Spectra and Force Constants for C4O4−2 and C5O5−2. J. Am. Chem. Soc. 1963, 85, 2580–2584. [Google Scholar] [CrossRef]
- Reis, L.V.; Serrano, J.P.; Almeida, P.; Santos, P.F. New Synthetic Approach to Aminosquarylium Cyanine Dyes. Synlett 2002, 2002, 1617–1620. [Google Scholar]
- Reis, L.; Serrano, J.; Almeida, P.; Santos, P. The synthesis and characterization of novel, aza-substituted squarylium cyanine dyes. Dyes Pigment. 2009, 81, 197–202. [Google Scholar] [CrossRef]
- Bricks, J.L.; Kachkovskii, A.D.; Slominskii, Y.L.; Gerasov, A.O.; Popov, S.V. Molecular design of near infrared polymethine dyes: A review. Dyes Pigment. 2015, 121, 238–255. [Google Scholar] [CrossRef]
- Xia, G.; Wang, H. Squaraine dyes: The hierarchical synthesis and its application in optical detection. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 84–113. [Google Scholar] [CrossRef]
- Avirah, R.R.; Jayaram, D.T.; Adarsh, N.; Ramaiah, D. Squaraine dyes in PDT: From basic design to In Vivo demonstration. Org. Biomol. Chem. 2012, 10, 911–920. [Google Scholar] [CrossRef]
- Ramaiah, D.; Joy, A.; Chandrasekhar, N.; Eldho, N.V.; Das, S.; George, M.V. Halogenated Squaraine Dyes as Potential Photochemotherapeutic Agents. Synthesis and Study of Photophysical Properties and Quantum Efficiencies of Singlet Oxygen Generation. Photochem. Photobiol. 1997, 65, 783–790. [Google Scholar] [CrossRef]
- Treibs, A.; Jacob, K. Cyclotrimethine Dyes Derived from Squaric Acid. Angew. Chem. Int. Ed. Engl. 1965, 4, 694. [Google Scholar] [CrossRef]
- Fan, B.; Maniglio, Y.; Simeunovic, M.; Kuster, S.; Geiger, T.; Hany, R.; Nüesch, F. Squaraine Planar-Heterojunction Solar Cells. Int. J. Photoenergy 2009, 2009, 1–7. [Google Scholar] [CrossRef]
- Park, J.; Viscardi, G.; Barolo, C.; Barbero, N. Near-infrared Sensitization in Dye-sensitized Solar Cells. CHIMIA Int. J. Chem. 2013, 67, 129–135. [Google Scholar] [CrossRef]
- Law, K.; Bailey, F.C. Squaraine chemistry. Synthesis and characterization of squaraine dyes having self-organizing properties. Dyes Pigment. 1992, 20, 25–40. [Google Scholar] [CrossRef]
- Law, K.Y. Organic photoconductive materials: Recent trends and developments. Chem. Rev. 1993, 93, 449–486. [Google Scholar] [CrossRef]
- Matsui, M.; Haishima, Y.; Kubota, Y.; Funabiki, K.; Jin, J.; Kim, T.H.; Manseki, K. Application of benz [c,d] indolenine-based unsymmetrical squaraine dyes to near-infrared dye-sensitized solar cells. Dyes Pigment. 2017, 141, 457–462. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, J.; Zhao, J.; Li, Y.; Qiao, B.; Song, D.; Huang, Y.; Xu, Z.; Zhao, S.; Xu, X. Improving the Charge Carrier Transport and Suppressing Recombination of Soluble Squaraine-Based Solar Cells via Parallel-Like Structure. Materials 2018, 11, 759. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Yang, L.; Zhang, J.; Liu, K.; Ding, L.; Peng, H.; Belfield, K.D.; Fang, Y. Squaraine-hydrazine adducts for fast and colorimetric detection of aldehydes in aqueous media. Sens. Actuators B Chem. 2019, 292, 88–93. [Google Scholar] [CrossRef]
- Lee, S.; Rao, B.A.; Son, Y.A. A highly selective fluorescent chemosensor for Hg2+ based on a squaraine–bis(rhodamine-B) derivative: Part II. Sens. Actuators B Chem. 2015, 210, 519–532. [Google Scholar] [CrossRef]
- Basheer, M.C.; Alex, S.; George Thomas, K.; Suresh, C.H.; Das, S. A squaraine-based chemosensor for Hg2+ and Pb2+. Tetrahedron 2006, 62, 605–610. [Google Scholar] [CrossRef]
- Ananda Rao, B.; Kim, H.; Son, Y.A. Synthesis of near-infrared absorbing pyrylium-squaraine dye for selective detection of Hg2+. Sens. Actuators B Chem. 2013, 188, 847–856. [Google Scholar] [CrossRef]
- Jin, B.; Zhang, X.; Zheng, W.; Liu, X.; Zhou, J.; Zhang, N.; Wang, F.; Shangguan, D. Dicyanomethylene-Functionalized Squaraine as a Highly Selective Probe for Parallel G-Quadruplexes. Anal. Chem. 2014, 86, 7063–7070. [Google Scholar] [CrossRef]
- Martins, T.D.; Pacheco, M.L.; Boto, R.E.; Almeida, P.; Farinha, J.P.S.; Reis, L.V. Synthesis, characterization and protein-association of dicyanomethylene squaraine dyes. Dyes Pigment. 2017, 147, 120–129. [Google Scholar] [CrossRef]
- Volkova, K.D.; Kovalska, V.B.; Losytskyy, M.Y.; Reis, L.V.; Santos, P.F.; Almeida, P.; Lynch, D.E.; Yarmoluk, S.M. Aza-substituted squaraines for the fluorescent detection of albumins. Dyes Pigment. 2011, 90, 41–47. [Google Scholar] [CrossRef]
- Lima, E.; Ferreira, O.; Gomes, V.S.D.; Santos, A.O.; Boto, R.E.; Fernandes, J.R.; Almeida, P.; Silvestre, S.M.; Reis, L.V. Synthesis and In Vitro evaluation of the antitumoral phototherapeutic potential of squaraine cyanine dyes derived from indolenine. Dyes Pigment. 2019, 167, 98–108. [Google Scholar] [CrossRef]
- Friães, S.; Silva, A.M.; Boto, R.E.; Ferreira, D.; Fernandes, J.R.; Souto, E.B.; Almeida, P.; Ferreira, L.F.V.; Reis, L.V. Synthesis, spectroscopic characterization and biological evaluation of unsymmetrical aminosquarylium cyanine dyes. Bioorg. Med. Chem. 2017, 25, 3803–3814. [Google Scholar] [CrossRef] [Green Version]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [Green Version]
- Parsa, N. Environmental factors inducing human cancers. Iran. J. Public Health 2012, 41, 1–9. [Google Scholar]
- Gilliam, L.A.A.; St. Clair, D.K. Chemotherapy-Induced Weakness and Fatigue in Skeletal Muscle: The Role of Oxidative Stress. Antioxid. Redox Signaling 2011, 15, 2543–2563. [Google Scholar] [CrossRef] [Green Version]
- Severino, P.; De Hollanda, L.M.; Santini, A.; Reis, L.V.; Souto, S.B.; Souto, E.B.; Silva, A.M. Advances in nanobiomaterials for oncology nanomedicine. In Nanobiomaterials in Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 91–115. ISBN 978-0-323-42863-7. [Google Scholar]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Buytaert, E.; Dewaele, M.; Agostinis, P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta (BBA) Rev. Cancer 2007, 1776, 86–107. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.F.; De Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. JCMT 2019, 5, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.; Khachemoune, A. An update on topical photodynamic therapy for clinical dermatologists. J. Dermatol. Treat. 2019, 30, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Blasi, M.A.; Pagliara, M.M.; Lanza, A.; Sammarco, M.G.; Caputo, C.G.; Grimaldi, G.; Scupola, A. Photodynamic Therapy in Ocular Oncology. Biomedicines 2018, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.M.; Adelzadeh, L.; Wu, J.J. Photodynamic therapy for psoriasis. J. Dermatol. Treat. 2015, 26, 202–207. [Google Scholar] [CrossRef]
- Silva, A.M.; Siopa, J.R.; Martins-Gomes, C.; Teixeira, M.C.; Santos, D.J.; Dos Anjos Pires, M.; Andreani, T. New strategies for the treatment of autoimmune diseases using nanotechnologies. In Emerging Nanotechnologies in Immunology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 135–163. ISBN 978-0-323-40016-9. [Google Scholar]
- Hatz, K.; Schneider, U.; Henrich, P.B.; Braun, B.; Sacu, S.; Prünte, C. Ranibizumab plus Verteporfin Photodynamic Therapy in Neovascular Age-Related Macular Degeneration: 12 Months of Retreatment and Vision Outcomes from a Randomized Study. Ophthalmologica 2014, 233, 66–73. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- Ciubini, B.; Visentin, S.; Serpe, L.; Canaparo, R.; Fin, A.; Barbero, N. Design and synthesis of symmetrical pentamethine cyanine dyes as NIR photosensitizers for PDT. Dyes Pigment. 2019, 160, 806–813. [Google Scholar] [CrossRef]
- Shen, Y.J.; Cao, J.; Sun, F.; Cai, X.L.; Li, M.M.; Zheng, N.N.; Qu, C.Y.; Zhang, Y.; Shen, F.; Zhou, M.; et al. Effect of photodynamic therapy with (17R,18R)-2-(1-hexyloxyethyl)-2-devinyl chlorine E6 trisodium salt on pancreatic cancer cells In Vitro and In Vivo. WJG 2018, 24, 5246–5258. [Google Scholar] [CrossRef]
- Mehraban, N.; Musich, P.; Freeman, H. Synthesis and Encapsulation of a New Zinc Phthalocyanine Photosensitizer into Polymeric Nanoparticles to Enhance Cell Uptake and Phototoxicity. Appl. Sci. 2019, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Jiang, C.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Amarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1996; ISBN 0-7506-3761-7. [Google Scholar]
- Pardal, A.; Ramos, S.; Santos, P.; Reis, L.; Almeida, P. Synthesis and Spectroscopic Characterisation of N-Alkyl Quaternary Ammonium Salts Typical Precursors of Cyanines. Molecules 2002, 7, 320–330. [Google Scholar] [CrossRef]
- Tatarets, A.; Fedyunyaeva, I.; Terpetschnig, E.; Patsenker, L. Synthesis of novel squaraine dyes and their intermediates. Dyes Pigment. 2005, 64, 125–134. [Google Scholar] [CrossRef]
- Kim, S.; Mor, G.K.; Paulose, M.; Varghese, O.K.; Baik, C.; Grimes, C.A. Molecular Design of Near-IR Harvesting Unsymmetrical Squaraine Dyes. Langmuir 2010, 26, 13486–13492. [Google Scholar] [CrossRef]
- Reynolds, K.; Young, D.; Loughlin, W. Limitations of the ‘Two-Phase’ Doebner-Miller Reaction for the Synthesis of Quinolines. Synthesis 2010, 2010, 3645–3648. [Google Scholar]
- Pacheco, M.L.F.M.; Friães, S.F.P.; Boto, R.E.; Almeida, P.; Silva, A.M.; Reis, L.V. Synthesis of a Squarylium Cyanine Dye as Potential Photosensitizer for Photodynamic Therapy (PDT). In Comprehensive Organic Chemistry Experiments for the Laboratory Classroom; The Royal Society of Chemistry: London, UK, 2017; pp. 301–305. ISBN 978-1-84973-963-4. [Google Scholar]
- Ferreira, D.P.; Conceição, D.S.; Calhelha, R.C.; Sousa, T.; Socoteanu, R.; Ferreira, I.C.F.R.; Vieira Ferreira, L.F. Porphyrin dye into biopolymeric chitosan films for localized photodynamic therapy of cancer. Carbohydr. Polym. 2016, 151, 160–171. [Google Scholar] [CrossRef]
- Andreani, T.; Kiill, C.P.; de Souza, A.L.R.; Fangueiro, J.F.; Fernandes, L.; Doktorovová, S.; Santos, D.L.; Garcia, M.L.; Gremião, M.P.D.; Souto, E.B.; et al. Surface engineering of silica nanoparticles for oral insulin delivery: Characterization and cell toxicity studies. Colloids Surf. B Biointerfaces 2014, 123, 916–923. [Google Scholar] [CrossRef] [Green Version]
- Severino, P.; Andreani, T.; Jäger, A.; Chaud, M.V.; Santana, M.H.A.; Silva, A.M.; Souto, E.B. Solid lipid nanoparticles for hydrophilic biotech drugs: Optimization and cell viability studies (Caco-2 & HEPG-2 cell lines). Eur. J. Med. Chem. 2014, 81, 28–34. [Google Scholar]
- Santos, P.F.; Reis, L.V.; Almeida, P.; Serrano, J.P.; Oliveira, A.S.; Vieira Ferreira, L.F. Efficiency of singlet oxygen generation of aminosquarylium cyanines. J. Photochem. Photobiol. A Chem. 2004, 163, 267–269. [Google Scholar] [CrossRef]
- Serpe, L.; Ellena, S.; Barbero, N.; Foglietta, F.; Prandini, F.; Gallo, M.P.; Levi, R.; Barolo, C.; Canaparo, R.; Visentin, S. Squaraines bearing halogenated moieties as anticancer photosensitizers: Synthesis, characterization and biological evaluation. Eur. J. Med. Chem. 2016, 113, 187–197. [Google Scholar] [CrossRef]
- Ferreira, D.P.; Conceição, D.S.; Ferreira, V.R.A.; Graça, V.C.; Santos, P.F.; Ferreira, L.F.V. Photochemical properties of squarylium cyanine dyes. Photochem. Photobiol. Sci. 2013, 12, 1948. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, Á.F.; Graça, V.C.; Calhelha, R.C.; Ferreira, I.C.F.R.; Santos, P.F. Aminosquaraines as potential photodynamic agents: Synthesis and evaluation of In Vitro cytotoxicity. Bioorg. Med. Chem. Lett. 2017, 27, 4467–4470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapozzi, V.; Beverina, L.; Salice, P.; Pagani, G.A.; Camerin, M.; Xodo, L.E. Photooxidation and Phototoxicity of π-Extended Squaraines. J. Med. Chem. 2010, 53, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.F.; Reis, L.V.; Almeida, P.; Oliveira, A.S.; Vieira Ferreira, L.F. Singlet oxygen generation ability of squarylium cyanine dyes. J. Photochem. Photobiol. A Chem. 2003, 160, 159–161. [Google Scholar] [CrossRef]
- Leir, C.M. An improvement in the Doebner-Miller synthesis of quinaldines. J. Org. Chem. 1977, 42, 911–913. [Google Scholar] [CrossRef]
- Lima, E.; Ferreira, O.; Silva, J.F.; Santos, A.O.; Boto, R.E.; Fernandes, J.R.; Almeida, P.; Silvestre, S.M.; Reis, L.V. Photodynamic activity of indolenine-based aminosquaraine cyanine dyes: Synthesis and In Vitro photobiological evaluation. Dyes Pigment. 2019, 108024. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef]
- Vankayala, R.; Hwang, K.C. Near-Infrared-Light-Activatable Nanomaterial-Mediated Phototheranostic Nanomedicines: An Emerging Paradigm for Cancer Treatment. Adv. Mater. 2018, 30, 1706320. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Arnaut, L.G. Photodynamic therapy (PDT) of cancer: From local to systemic treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef]
- van Straten, D.; Mashayekhi, V.; de Bruijn, H.; Oliveira, S.; Robinson, D. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Paszko, E.; Ehrhardt, C.; Senge, M.O.; Kelleher, D.P.; Reynolds, J.V. Nanodrug applications in photodynamic therapy. Photodiagn. Photodyn. Ther. 2011, 8, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I. Singlet oxygen in biological systems. Trends Biochem. Sci. 1977, 2, 35–38. [Google Scholar] [CrossRef]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moan, J. Effect of bleaching of porphyrin sensitizers during photodynamic therapy. Cancer Lett. 1986, 33, 45–53. [Google Scholar] [CrossRef]
- Yogo, T.; Urano, Y.; Ishitsuka, Y.; Maniwa, F.; Nagano, T. Highly Efficient and Photostable Photosensitizer Based on BODIPY Chromophore. J. Am. Chem. Soc. 2005, 127, 12162–12163. [Google Scholar] [CrossRef]
- Ferreira, J.; Menezes, P.F.C.; Sibata, C.H.; Allison, R.R.; Zucoloto, S.; Castro e Silva, O.; Bagnato, V.S. Can efficiency of the photosensitizer be predicted by its photostability in solution? Laser Phys. 2009, 19, 1932–1938. [Google Scholar] [CrossRef]
- Terpetschnig, E.; Szmacinski, H.; Lakowicz, J.R. An investigation of squaraines as a new class of fluorophores with long-wavelength excitation and emission. J. Fluoresc. 1993, 3, 153–155. [Google Scholar] [CrossRef]
- Donato, M.T.; Tolosa, L.; Gómez-Lechón, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. In Protocols in In Vitro Hepatocyte Research; Vinken, M., Rogiers, V., Eds.; Springer: New York, NY, USA, 2015; Volume 1250, pp. 77–93. ISBN 978-1-4939-2073-0. [Google Scholar]
- Granados-Romero, J.J.; Valderrama-Treviño, A.I.; Contreras-Flores, E.H.; Barrera-Mera, B.; Herrera Enríquez, M.; Uriarte-Ruíz, K.; Ceballos-Villalba, J.C.; Estrada-Mata, A.G.; Alvarado Rodríguez, C.; Arauz-Peña, G. Colorectal cancer: A review. Int. J. Res. Med. Sci. 2017, 5, 4667. [Google Scholar] [CrossRef] [Green Version]
- Chantret, I.; Barbat, A.; Dussaulx, E.; Brattain, M.G.; Zweibaum, A. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: A survey of twenty cell lines. Cancer Res. 1988, 48, 1936–1942. [Google Scholar]
- Hamid, R.; Rotshteyn, Y.; Rabadi, L.; Parikh, R.; Bullock, P. Comparison of alamar blue and MTT assays for high through-put screening. Toxicol. In Vitro 2004, 18, 703–710. [Google Scholar] [CrossRef]
- International Standard (ISO). Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity, 3rd ed.; ISO-10993-5; International Standard (ISO): Geneva, Switzerland, 2009. [Google Scholar]
- Bácskay, I.; Nemes, D.; Fenyvesi, F.; Váradi, J.; Vasvári, G.; Fehér, P.; Vecsernyés, M.; Ujhelyi, Z. Role of Cytotoxicity Experiments in Pharmaceutical Development. In Cytotoxicity; Çelik, T.A., Ed.; InTech: London, UK, 2018; ISBN 978-1-78923-430-5. [Google Scholar]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, P.F.; Reis, L.V.; Duarte, I.; Serrano, J.P.; Almeida, P.; Oliveira, A.S.; Ferreira, L.F.V. Synthesis and Photochemical Evaluation of Iodinated Squarylium Cyanine Dyes. HCA 2005, 88, 1135–1143. [Google Scholar] [CrossRef]






| Dye | DMSO | DMEM | ΦΔ | |
|---|---|---|---|---|
| λmax (nm) | log ε (M−1·cm−1) | λmax (nm) | ||
| 10 | 726 | 5.26 | 640 | 0.03 |
| 11 | 677 | 5.25 | – | – |
| 12 | 705 | 5.17 | 619 | 0.03 |
| 13 | 715 | 5.18 | 637 | 0.05 |
| Solvent | Dye 10 | Dye 11 | Dye 12 | Dye 13 | ||||
|---|---|---|---|---|---|---|---|---|
| λmax | log ε | λmax | log ε | λmax | log ε | λmax | log ε | |
| ACN | 714 | 5.25 | 665 | 5.28 | 686 | 5.36 | 699 | 5.25 |
| ACT | 720 | 5.12 | 668 | 5.24 | 690 | 5.36 | 703 | 5.21 |
| DCM | 723 | 5.16 | 672 | 5.33 | 694 | 5.38 | 707 | 5.30 |
| DMF | 725 | 5.14 | 675 | 5.23 | 700 | 5.31 | 711 | 5.20 |
| DXN | 729 | 5.15 | 680 | 5.11 | 706 | 5.27 | 716 | 5.15 |
| EtOH | 701 | 5.22 | 668 | 5.27 | 693 | 5.38 | 702 | 5.28 |
| MeOH | 695 | 4.45 | 665 | 4.60 | 687 | 5.36 | 698 | 5.25 |
| THF | 733 | 5.06 | 671 | 5.26 | 702 | 5.26 | 714 | 5.17 |
| Dye | Irradiation Time | Caco-2 | HepG2 | ||
|---|---|---|---|---|---|
| 1 h | 24 h | 1 h | 24 h | ||
| 10 | 0′ | >10 | 8.811 | >10 | >10 |
| 7′ | 2.277 | 2.320 | 9.606 | 5.068 | |
| 14′ | 1.655 | 0.456 | 3.291 | 2.306 | |
| 12 | 0′ | >10 | 4.383 | 4.491 | 2.116 |
| 7′ | 1.497 | 1.069 | 1.478 | 1.182 | |
| 14′ | 1.322 | 0.989 | 1.145 | 0.903 | |
| 13 | 0′ | 9.632 | 3.411 | 2.316 | 1.645 |
| 7′ | 1.973 | 1.256 | 1.414 | 0.800 | |
| 14′ | 1.567 | 0.751 | 1.441 | 0.732 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friães, S.; Lima, E.; Boto, R.E.; Ferreira, D.; Fernandes, J.R.; Ferreira, L.F.V.; Silva, A.M.; Reis, L.V. Photophysicochemical Properties and In Vitro Phototherapeutic Effects of Iodoquinoline- and Benzothiazole-Derived Unsymmetrical Squaraine Cyanine Dyes. Appl. Sci. 2019, 9, 5414. https://doi.org/10.3390/app9245414
Friães S, Lima E, Boto RE, Ferreira D, Fernandes JR, Ferreira LFV, Silva AM, Reis LV. Photophysicochemical Properties and In Vitro Phototherapeutic Effects of Iodoquinoline- and Benzothiazole-Derived Unsymmetrical Squaraine Cyanine Dyes. Applied Sciences. 2019; 9(24):5414. https://doi.org/10.3390/app9245414
Chicago/Turabian StyleFriães, Sofia, Eurico Lima, Renato E. Boto, Diana Ferreira, José R. Fernandes, Luis F. V. Ferreira, Amélia M. Silva, and Lucinda V. Reis. 2019. "Photophysicochemical Properties and In Vitro Phototherapeutic Effects of Iodoquinoline- and Benzothiazole-Derived Unsymmetrical Squaraine Cyanine Dyes" Applied Sciences 9, no. 24: 5414. https://doi.org/10.3390/app9245414
APA StyleFriães, S., Lima, E., Boto, R. E., Ferreira, D., Fernandes, J. R., Ferreira, L. F. V., Silva, A. M., & Reis, L. V. (2019). Photophysicochemical Properties and In Vitro Phototherapeutic Effects of Iodoquinoline- and Benzothiazole-Derived Unsymmetrical Squaraine Cyanine Dyes. Applied Sciences, 9(24), 5414. https://doi.org/10.3390/app9245414