Immersive Virtual Reality in Stroke Patients as a New Approach for Reducing Postural Disabilities and Falls Risk: A Case Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects Selection
2.2.1. Patient 1
2.2.2. Patient 2
2.2.3. Patient 3
2.3. Intervention Protocol
2.4. Conventional Physiotherapy
2.5. Study Variables
3. Results
3.1. Postural Balance
3.2. Gait
3.3. Risk of falls
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.B.; Culebras, A.; Elkind, M.S.V.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnan, G.A.; Fisher, M.; Macleod, M.; Davis, S.M. Stroke. Lancet 2008, 371, 1612–1623. [Google Scholar] [CrossRef]
- De Luca, R.; Manuli, A.; De Domenico, C.; Lo Voi, E.; Buda, A.; Maresca, G.; Bramanti, A.; Calabro, R.S. Improving neuropsychiatric symptoms following stroke using virtual reality: A case report. Medicine 2019, 98, e15236. [Google Scholar] [CrossRef] [PubMed]
- Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [CrossRef] [Green Version]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Li, K.; Wei, N.; Yin, C.; Yue, S. Evaluation of postural instability in stroke patient during quiet standing. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, South Korea, 11–15 July 2017; pp. 2522–2525. [Google Scholar]
- Li, J.; Zhong, D.; Ye, J.; He, M.; Liu, X.; Zheng, H.; Jin, R.; Zhang, S.-L. Rehabilitation for balance impairment in patients after stroke: A protocol of a systematic review and network meta-analysis. BMJ Open 2019, 9, e026844. [Google Scholar] [CrossRef] [Green Version]
- Bronstein, A.M. The interaction of otolith and proprioceptive information in the perception of verticality. The effects of labyrinthine and CNS disease. Ann. N. Y. Acad. Sci. 1999, 871, 324–333. [Google Scholar] [CrossRef]
- Alyono, J.C. Vertigo and Dizziness: Understanding and Managing Fall Risk. Otolaryngol. Clin. N. Am. 2018, 51, 725–740. [Google Scholar] [CrossRef]
- Yuan, Z.-C.; Mo, H.; Guan, J.; He, J.-L.; Wu, Z.-J. Risk of hip fracture following stroke, a meta-analysis of 13 cohort studies. Osteoporos. Int. 2016, 27, 2673–2679. [Google Scholar] [CrossRef]
- Kobayashi, K.; Imagama, S.; Inagaki, Y.; Suzuki, Y.; Ando, K.; Nishida, Y.; Nagao, Y.; Ishiguro, N. Incidence and characteristics of accidental falls in hospitalizations. Nagoya J. Med. Sci. 2017, 79, 291–298. [Google Scholar]
- Kobayashi, K.; Ando, K.; Inagaki, Y.; Suzuki, Y.; Nagao, Y.; Ishiguro, N.; Imagama, S. Characteristics of falls in orthopedic patients during hospitalization. Nagoya J. Med. Sci. 2018, 80, 341–349. [Google Scholar]
- Kim, J.H.; Jang, S.H.; Kim, C.S.; Jung, J.H.; You, J.H. Use of virtual reality to enhance balance and ambulation in chronic stroke: A double-blind, randomized controlled study. Am. J. Phys. Med. Rehabil. 2009, 88, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, Y.J.; Park, S.W.; De Luca, R.; Manuli, A.; De Domenico, C.; Lo Voi, E.; Buda, A.; Maresca, G.; Bramanti, A.; et al. The Effects of Virtual Reality Training on Function in Chronic Stroke Patients: A Systematic Review and Meta-Analysis. Medicine 2019, 2019, e15236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Marcos, D. Virtual reality experiences, embodiment, videogames and their dimensions in neurorehabilitation. J. Neuroeng. Rehabil. 2018, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Lin, Q.; Lo, W.-L.; Mao, Y.-R.; Shi, X.-C.; Cates, R.S.; Zhou, S.-F.; Huang, D.-F.; Li, L. Cerebral Reorganization in Subacute Stroke Survivors after Virtual Reality-Based Training: A Preliminary Study. Behav. Neurol. 2017, 2017, 6261479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbetta, D.; Imeri, F.; Gatti, R. Rehabilitation that incorporates virtual reality is more effective than standard rehabilitation for improving walking speed, balance and mobility after stroke: A systematic review. J. Physiother. 2015, 61, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheehy, L.; Taillon-Hobson, A.; Sveistrup, H.; Bilodeau, M.; Yang, C.; Finestone, H. Sitting Balance Exercise Performed Using Virtual Reality Training on a Stroke Rehabilitation Inpatient Service: A Randomized Controlled Study. PM R 2020. [Google Scholar] [CrossRef]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, R.; Semnani, A.V.; Mirmohammadkhani, M.; Grampurohit, N. Effects of Virtual Reality Compared to Conventional Therapy on Balance Poststroke: A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2019, 28, 1787–1798. [Google Scholar] [CrossRef]
- Golla, A.; Muller, T.; Wohlfarth, K.; Jahn, P.; Mattukat, K.; Mau, W. Home-based balance training using Wii Fit: A pilot randomised controlled trial with mobile older stroke survivors. Pilot Feasibility Stud. 2018, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Munoz, C.; Casuso-Holgado, M.J. Effectiveness of Wii Fit Balance board in comparison with other interventions for post-stroke balance rehabilitation. Systematic review and meta-analysis. Rev. Neurol. 2019, 69, 271–279. [Google Scholar] [PubMed]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef] [PubMed] [Green Version]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83 (Suppl. 2), S7–S11. [Google Scholar] [PubMed]
- Hayes, K.W.; Johnson, M.E. Measures of adult general performance tests: The Berg Balance Scale, Dynamic Gait Index (DGI), Gait Velocity, Physical Performance Test (PPT), Timed Chair Stand Test, Timed Up and Go, and Tinetti Performance-Oriented Mobility Assessment (POMA). Arthritis Care Res. 2003, 49, S28–S42. [Google Scholar] [CrossRef]
- Rodríguez Guevara, C.; Lugo, L.H. Validity and reliability of Tinetti Scale for Colombian people. Rev. Colomb. Reumatol. 2012, 19, 218–233. [Google Scholar]
- Berg, K.; Wood-Dauphinee, S.; Williams, J.I. The Balance Scale: Reliability assessment with elderly residents and patients with an acute stroke. Scand. J. Rehabil. Med. 1995, 27, 27–36. [Google Scholar]
- Madhavan, S.; Bishnoi, A. Comparison of the Mini-Balance Evaluations Systems Test with the Berg Balance Scale in relationship to walking speed and motor recovery post stroke. Top. Stroke Rehabil. 2017, 24, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Negrillo-Cardenas, J.; Rueda-Ruiz, A.J.; Ogayar-Anguita, C.J.; Lomas-Vega, R.; Segura-Sanchez, R.J. A System for the Measurement of the Subjective Visual Vertical using a Virtual Reality Device. J. Med. Syst. 2018, 42, 124. [Google Scholar] [CrossRef]
- Piscicelli, C.; Perennou, D. Visual verticality perception after stroke: A systematic review of methodological approaches and suggestions for standardization. Ann. Phys. Rehabil. Med. 2017, 60, 208–216. [Google Scholar] [CrossRef]
- Sawacha, Z.; Carraro, E.; Contessa, P.; Guiotto, A.; Masiero, S.; Cobelli, C. Relationship between clinical and instrumental balance assessments in chronic post-stroke hemiparesis subjects. J. Neuroeng. Rehabil. 2013, 10, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonan, I.V.; Guettard, E.; Leman, M.C.; Colle, F.M.; Yelnik, A.P. Subjective Visual Vertical Perception Relates to Balance in Acute Stroke. Arch. Phys. Med. Rehabil. 2006, 87, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, T.; Iosa, M.; Morone, G.; Fratte, M.D.; Paolucci, S.; Saraceni, V.M.; Villani, C. Romberg ratio coefficient in quiet stance and postural control in Parkinson’s disease. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2018, 39, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.S.; Hui-Chan, C.W. The timed up & go test: Its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch. Phys. Med. Rehabil. 2005, 86, 1641–1647. [Google Scholar]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Montilla-Ibanez, A.; Martinez-Amat, A.; Lomas-Vega, R.; Cruz-Diaz, D.; la Torre-Cruz, M.J.D.; Casuso-Perez, R.; Hita-Contreras, F. The Activities-specific Balance Confidence scale: Reliability and validity in Spanish patients with vestibular disorders. Disabil. Rehabil. 2017, 39, 697–703. [Google Scholar] [CrossRef]
- Yardley, L.; Beyer, N.; Hauer, K.; Kempen, G.; Piot-Ziegler, C.; Todd, C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005, 34, 614–619. [Google Scholar] [CrossRef] [Green Version]
- Salbach, N.M.; Mayo, N.E.; Robichaud-Ekstrand, S.; Hanley, J.A.; Richards, C.L.; Wood-Dauphinee, S. Balance self-efficacy and its relevance to physical function and perceived health status after stroke. Arch. Phys. Med. Rehabil. 2006, 87, 364–370. [Google Scholar] [CrossRef]
- Park, E.-Y.; Lee, Y.-J.; Choi, Y.-I. The sensitivity and specificity of the Falls Efficacy Scale and the Activities-specific Balance Confidence Scale for hemiplegic stroke patients. J. Phys. Ther. Sci. 2018, 30, 741–743. [Google Scholar] [CrossRef] [Green Version]
- Kempen, G.I.J.M.; Todd, C.J.; Van Haastregt, J.C.M.; Zijlstra, G.A.R.; Beyer, N.; Freiberger, E.; Hauer, K.A.; Piot-Ziegler, C.; Yardley, L. Cross-cultural validation of the Falls Efficacy Scale International (FES-I) in older people: Results from Germany, the Netherlands and the UK were satisfactory. Disabil. Rehabil. 2007, 29, 155–162. [Google Scholar] [CrossRef]
- Buurke, J.H.; Nene, A.V.; Kwakkel, G.; Erren-Wolters, V.; Ijzerman, M.J.; Hermens, H.J. Recovery of gait after stroke: What changes? Neurorehabil. Neural Repair 2008, 22, 676–683. [Google Scholar] [CrossRef] [PubMed]
- de Paula, G.V.; da Silva, T.R.; de Souza, J.T.; Luvizutto, G.J.; Bazan, S.G.Z.; Modolo, G.P.; Winckler, F.C.; de Oliveira Antunes, L.C.; Martin, L.C.; da Costa, R.D.M.; et al. Effect of ankle-foot orthosis on functional mobility and dynamic balance of patients after stroke: Study protocol for a randomized controlled clinical trial. Medicine 2019, 98, e17317. [Google Scholar] [CrossRef] [PubMed]
- Volgger, V.; Gurkov, R. Acute vestibular syndrome in cerebellar stroke: A case report and review of the literature. HNO 2017, 65, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Molina, F.; Lomas-Vega, R.; Obrero-Gaitán, E.; Rus, A.; Almagro, D.R.; Del-Pino-Casado, R. Misperception of the subjective visual vertical in neurological patients with or without stroke: A meta-analysis. NeuroRehabilitation 2019, 44, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, J.A.; Kumar, S.; Sundaram, A.N. Ocular torsion and vertical misalignment. Curr. Opin. Neurol. 2011, 24, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.R.; Hyngstrom, A.S.; Schmit, B.D. Influence of visual feedback on dynamic balance control in chronic stroke survivors. J. Biomech. 2016, 49, 698–703. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.; Park, Y.; Lee, B.-H. Effects of community-based virtual reality treadmill training on balance ability in patients with chronic stroke. J. Phys. Ther. Sci. 2015, 27, 655–658. [Google Scholar] [CrossRef] [Green Version]
- Tsur, A.; Segal, Z. Falls in stroke patients: Risk factors and risk management. Isr. Med. Assoc. J. 2010, 12, 216–219. [Google Scholar]
- Dieterich, M.; Brandt, T. Perception of Verticality and Vestibular Disorders of Balance and Falls. Front. Neurol. 2019, 10, 172. [Google Scholar] [CrossRef] [Green Version]
- Hara, Y. Brain plasticity and rehabilitation in stroke patients. J. Nippon Med. Sch. 2015, 82, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Garrett, B.; Taverner, T.; Gromala, D.; Tao, G.; Cordingley, E.; Sun, C. Virtual Reality Clinical Research: Promises and Challenges. JMIR Serious Games 2018, 6, e10839. [Google Scholar] [CrossRef] [PubMed]
- Schuemie, M.J.; van der Straaten, P.; Krijn, M.; van der Mast, C.A. Research on presence in virtual reality: A survey. Cyberpsychol. Behav. 2001, 4, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Lim, J.-H.; Jeon, B.-H.; Song, C.-S. Non-immersive Virtual Reality Rehabilitation Applied to a Task-oriented Approach for Stroke Patients: A Randomized Controlled Trial. Restor. Neurol. Neurosci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Saposnik, G.; Cohen, L.G.; Mamdani, M.; Pooyania, S.; Ploughman, M.; Cheung, D.; Shaw, J.; Hall, J.; Nord, P.; Dukelow, S.; et al. Efficacy and safety of non-immersive virtual reality exercising in stroke rehabilitation (EVREST): A randomised, multicentre, single-blind, controlled trial. Lancet Neurol. 2016, 15, 1019–1027. [Google Scholar] [CrossRef] [Green Version]
- Rougier, P.R.; Perennou, D. Upright standing after stroke: How loading-unloading mechanism participates to the postural stabilization. Hum. Mov. Sci. 2019, 64, 47–54. [Google Scholar] [CrossRef]
- Perennou, D.; Piscicelli, C.; Barbieri, G.; Jaeger, M.; Marquer, A.; Barra, J. Measuring verticality perception after stroke: Why and how? Neurophysiol. Clin. 2014, 44, 25–32. [Google Scholar] [CrossRef]
- Brandt, T.; Dieterich, M. Vestibular syndromes in the roll plane: Topographic diagnosis from brainstem to cortex. Ann. Neurol. 1994, 36, 337–347. [Google Scholar] [CrossRef]
- Witsch, J.; Ferrer, M.; Navaratnam, D. Teaching Video NeuroImages: Vestibulo-ocular reflex defect in cerebellar stroke. Neurology 2018, 91, e888–e889. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.M.; Klein, C.S.; Suresh, N.L.; Rymer, W.Z. Asymmetries in vestibular evoked myogenic potentials in chronic stroke survivors with spastic hypertonia: Evidence for a vestibulospinal role. Clin. Neurophysiol. 2014, 125, 2070–2078. [Google Scholar] [CrossRef] [Green Version]
- Della Casa, E.; Affolter Helbling, J.; Meichtry, A.; Luomajoki, H.; Kool, J. Head-eye movement control tests in patients with chronic neck pain; inter-observer reliability and discriminative validity. BMC Musculoskelet. Disord. 2014, 15, 16. [Google Scholar] [CrossRef] [Green Version]
- Naranjo, E.N.; Cleworth, T.W.; Allum, J.H.J.; Inglis, J.T.; Lea, J.; Westerberg, B.D.; Carpenter, M.G. Vestibulo-spinal and vestibulo-ocular reflexes are modulated when standing with increased postural threat. J. Neurophysiol. 2016, 115, 833–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arntz, A.I.; van der Putte, D.A.M.; Jonker, Z.D.; Hauwert, C.M.; Frens, M.A.; Forbes, P.A. The Vestibular Drive for Balance Control Is Dependent on Multiple Sensory Cues of Gravity. Front. Physiol. 2019, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, N.A.; Almeida, M.G.R.; Pompeu, J.E.; Polese, J.C.; Torriani-Pasin, C. A combination of multimodal physical exercises in real and virtual environments for individuals after chronic stroke: Study protocol for a randomized controlled trial. Trials 2019, 20, 436. [Google Scholar] [CrossRef] [PubMed]
- Kannan, L.; Vora, J.; Bhatt, T.; Hughes, S.L. Cognitive-motor exergaming for reducing fall risk in people with chronic stroke: A randomized controlled trial. NeuroRehabilitation 2019, 44, 493–510. [Google Scholar] [CrossRef] [PubMed]
| CHARACTERISTICS | Participant 1 | Participant 2 | Participant 3 | |
|---|---|---|---|---|
| Socio-demographic | Age | 45 | 50 | 53 |
| Gender | Male | Male | Male | |
| Weigh (kg) | 73 | 84 | 65 | |
| Height (cm) | 175 | 179 | 177 | |
| Body Max Index | 23.8 | 26.2 | 20.8 | |
| Marital status | Married | Married | Divorced | |
| Education level | University | Primary | Primary | |
| Prior comorbidity | Hypertension | Yes | Yes | Yes |
| Hypercholesterolemia | No | No | Yes | |
| Diabetes mellitus | No | Yes | No | |
| Vertigo | Yes | No | No | |
| Falls | Yes | Yes | Yes | |
| Number of falls * | 2 | 3 | 1 | |
| Injuring falls | Yes | Yes | Yes | |
| Type of injury | Hematoma | Wounds | Bone fracture | |
| Diagnosis | Stroke type | Ischemic | Ischemic | Ischemic |
| Stroke localization | Left | Left | Left | |
| Hemiplegia | Yes | Yes | Yes | |
| Side | Right | Right | Right | |
| Evolution | 6 months | 9 months | 10 months | |
| Assigned intervention | Immersive VR | CT | NI | |
| VARIABLES | OUTCOMES | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INTEREST VARIABLE | RATING SCALE | P1 (VR) | P2 (CT) | P3 (NT) | |||||||||
| T0 | T1 | % var | Clin Imp. | T0 | T1 | % var | Clin Imp. | T0 | T1 | % var | Clin Imp. | ||
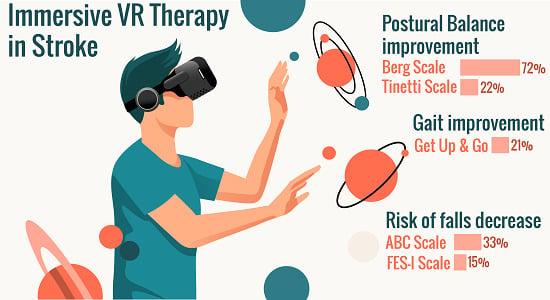
| BALANCE | BBS | 28 | 34 | 22 | Yes | 15 | 18 | 20 | Yes | 8 | 7 | 12 | No |
| Tinetti | 11 | 19 | 72 | Yes | 7 | 9 | 29 | Yes | 6 | 6 | 0 | No | |
| VESTIBULAR | SVV | 5.75 ± 1.25 | 4 ± 1.2 | 30 | Yes | 5.2 ± 2.7 | 4.8 ± 1.8 | 7 | Yes | 3.7 ± 2 | 3.4 ± 1.67 | 8 | Yes |
| Romberg | 427 | 398 | 7 | Yes | 192 | 226 | 17 | Yes | NP | NP | NP | NP | |
| FALLS | ABC | 24 | 32 | 33 | Yes | 13 | 15 | 2 | Yes | 4 | 4 | 0 | No |
| FES-I | 47 | 40 | 15 | Yes | 59 | 54 | 8 | Yes | 61 | 60 | 2 | Yes | |
| GAIT | TGUGT | 29 | 23 | 21 | Yes | 33 | 31 | 7 | Yes | NP | NP | NP | NP |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Pérez, I.; Nieto-Escamez, F.A.; Obrero-Gaitán, E. Immersive Virtual Reality in Stroke Patients as a New Approach for Reducing Postural Disabilities and Falls Risk: A Case Series. Brain Sci. 2020, 10, 296. https://doi.org/10.3390/brainsci10050296
Cortés-Pérez I, Nieto-Escamez FA, Obrero-Gaitán E. Immersive Virtual Reality in Stroke Patients as a New Approach for Reducing Postural Disabilities and Falls Risk: A Case Series. Brain Sciences. 2020; 10(5):296. https://doi.org/10.3390/brainsci10050296
Chicago/Turabian StyleCortés-Pérez, Irene, Francisco Antonio Nieto-Escamez, and Esteban Obrero-Gaitán. 2020. "Immersive Virtual Reality in Stroke Patients as a New Approach for Reducing Postural Disabilities and Falls Risk: A Case Series" Brain Sciences 10, no. 5: 296. https://doi.org/10.3390/brainsci10050296
APA StyleCortés-Pérez, I., Nieto-Escamez, F. A., & Obrero-Gaitán, E. (2020). Immersive Virtual Reality in Stroke Patients as a New Approach for Reducing Postural Disabilities and Falls Risk: A Case Series. Brain Sciences, 10(5), 296. https://doi.org/10.3390/brainsci10050296