Can Pallidal Deep Brain Stimulation Rescue Borderline Dystonia? Possible Coexistence of Functional (Psychogenic) and Organic Components
Abstract
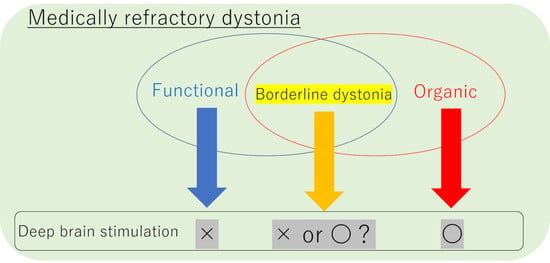
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Assessment Instruments
2.3. Surgical Procedure
3. Results
3.1. Clinical Characteristics of the Dystonia Patients
3.2. Electrophysiological Findings and Postoperative Verification of the Implanted Electrodes
3.3. Stimulation Settings
3.4. Assessment of Symptoms after the Surgery
3.5. Comparison with Other Types of Dystonia
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hallett, M. Functional movement disorders: Is the crisis resolved? Mov. Disord. 2019, 34, 971–974. [Google Scholar] [CrossRef]
- Jinnah, H.A.; Factor, S.A. Diagnosis and treatment of dystonia. Neurol. Clin. 2015, 33, 77–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albanese, A.; Asmus, F.; Bhatia, K.P.; Elia, A.E.; Elibol, B.; Filippini, G.; Gasser, T.; Krauss, J.K.; Nardocci, N.; Newton, A.; et al. Efns guidelines on diagnosis and treatment of primary dystonias. Eur. J. Neurol. 2011, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.L.; Bressman, S.B. The diagnosis of dystonia. Lancet Neurol. 2006, 5, 780–790. [Google Scholar] [CrossRef]
- Albanese, A.; Lalli, S. Is this dystonia? Mov. Disord. 2009, 24, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Kaji, R.; Hasegawa, K.; Ugawa, Y.; Osawa, M.; Kashihara, K.; Kawarai, T.; Kobayashi, T.; Sakamoto, T.; Taira, T.; Tamagawa, S.; et al. Practical Guideline for Dystonia 2018, 1st ed.; Nankodo Co. Ltd.: Tokyo, Japan, 2018. [Google Scholar]
- Edwards, M.J.; Bhatia, K.P. Functional (psychogenic) movement disorders: Merging mind and brain. Lancet Neurol. 2012, 11, 250–260. [Google Scholar] [CrossRef]
- Stone, J.; Carson, A.; Duncan, R.; Roberts, R.; Warlow, C.; Hibberd, C.; Coleman, R.; Cull, R.; Murray, G.; Pelosi, A.; et al. Who is referred to neurology clinics?--the diagnoses made in 3781 new patients. Clin. Neurol. Neurosurg. 2010, 112, 747–751. [Google Scholar] [CrossRef]
- Anderson, K.E.; Gruber-Baldini, A.L.; Vaughan, C.G.; Reich, S.G.; Fishman, P.S.; Weiner, W.J.; Shulman, L.M. Impact of psychogenic movement disorders versus parkinson’s on disability, quality of life, and psychopathology. Mov. Disord. 2007, 22, 2204–2209. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (dsm-5); American Psychiatric Press Inc.: Washington, DC, USA, 2013. [Google Scholar]
- Baizabal-Carvallo, J.F.; Hallett, M.; Jankovic, J. Pathogenesis and pathophysiology of functional (psychogenic) movement disorders. Neurobiol. Dis. 2019, 127, 32–44. [Google Scholar] [CrossRef]
- Factor, S.A.; Podskalny, G.D.; Molho, E.S. Psychogenic movement disorders: Frequency, clinical profile, and characteristics. J. Neurol. Neurosurg. Psychiatry 1995, 59, 406–412. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Lang, A.E. Psychogenic movement disorders. Curr. Opin. Neurol. 2009, 22, 430–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrag, A.; Trimble, M.; Quinn, N.; Bhatia, K. The syndrome of fixed dystonia: An evaluation of 103 patients. Brain 2004, 127, 2360–2372. [Google Scholar] [CrossRef]
- Bhatia, K.P.; Bhatt, M.H.; Marsden, C.D. The causalgia-dystonia syndrome. Brain 1993, 116, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Ganos, C.; Edwards, M.J.; Bhatia, K.P. The phenomenology of functional (psychogenic) dystonia. Mov. Disord. Clin. Pract. 2014, 1, 36–44. [Google Scholar] [CrossRef]
- Kranick, S.M.; Gorrindo, T.; Hallett, M. Psychogenic movement disorders and motor conversion: A roadmap for collaboration between neurology and psychiatry. Psychosomatics 2011, 52, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgante, F.; Edwards, M.J.; Espay, A.J.; Fasano, A.; Mir, P.; Martino, D.; DISMOV-SIN Study Group on Psychogenic Movement Disorders. Diagnostic agreement in patients with psychogenic movement disorders. Mov. Disord. 2012, 27, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Shamy, M.C. The treatment of psychogenic movement disorders with suggestion is ethically justified. Mov. Disord. 2010, 25, 260–264. [Google Scholar] [CrossRef]
- Avanzino, L.; Martino, D.; van de Warrenburg, B.P.; Schneider, S.A.; Abbruzzese, G.; Defazio, G.; Schrag, A.; Bhatia, K.P.; Rothwell, J.C. Cortical excitability is abnormal in patients with the “fixed dystonia” syndrome. Mov. Disord. 2008, 23, 646–652. [Google Scholar] [CrossRef]
- Espay, A.J.; Morgante, F.; Purzner, J.; Gunraj, C.A.; Lang, A.E.; Chen, R. Cortical and spinal abnormalities in psychogenic dystonia. Ann. Neurol. 2006, 59, 825–834. [Google Scholar] [CrossRef]
- Kobayashi, K.; Lang, A.E.; Hallett, M.; Lenz, F.A. Thalamic neuronal and emg activity in psychogenic dystonia compared with organic dystonia. Mov. Disord. 2011, 26, 1348–1352. [Google Scholar] [CrossRef]
- Ramos, V.F.; Pillai, A.S.; Lungu, C.; Ostrem, J.; Starr, P.; Hallett, M. Intraoperative neurophysiology in deep brain surgery for psychogenic dystonia. Ann. Clin. Transl. Neurol. 2015, 2, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, I.N.; Tomic, A.; Voncina, M.M.; Pesic, D.; Kostic, V.S. Characteristics of two distinct clinical phenotypes of functional (psychogenic) dystonia: Follow-up study. J. Neurol. 2018, 265, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Romito, L.M.; Franzini, A.; Perani, D.; Carella, F.; Marras, C.; Capus, L.; Garibotto, V.; Broggi, G.; Albanese, A. Fixed dystonia unresponsive to pallidal stimulation improved by motor cortex stimulation. Neurology 2007, 68, 875–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauls, K.A.M.; Krauss, J.K.; Kampfer, C.E.; Kuhn, A.A.; Schrader, C.; Sudmeyer, M.; Allert, N.; Benecke, R.; Blahak, C.; Boller, J.K.; et al. Causes of failure of pallidal deep brain stimulation in cases with pre-operative diagnosis of isolated dystonia. Parkinsonism Relat. Disord. 2017, 43, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Batshaw, M.L.; Wachtel, R.C.; Deckel, A.W.; Whitehouse, P.J.; Moses, H., 3rd; Fochtman, L.J.; Eldridge, R. Munchausen’s syndrome simulating torsion dystonia. N. Engl. J. Med. 1985, 312, 1437–1439. [Google Scholar] [CrossRef]
- Lempert, T.; Dieterich, M.; Huppert, D.; Brandt, T. Psychogenic disorders in neurology: Frequency and clinical spectrum. Acta Neurol. Scand. 1990, 82, 335–340. [Google Scholar] [CrossRef]
- Burke, R.E.; Fahn, S.; Marsden, C.D.; Bressman, S.B.; Moskowitz, C.; Friedman, J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 1985, 35, 73–77. [Google Scholar] [CrossRef] [Green Version]
- Sako, W.; Goto, S.; Shimazu, H.; Murase, N.; Matsuzaki, K.; Tamura, T.; Mure, H.; Tomogane, Y.; Arita, N.; Yoshikawa, H.; et al. Bilateral deep brain stimulation of the globus pallidus internus in tardive dystonia. Mov. Disord. 2008, 23, 1929–1931. [Google Scholar] [CrossRef]
- Sako, W.; Morigaki, R.; Mizobuchi, Y.; Tsuzuki, T.; Ima, H.; Ushio, Y.; Nagahiro, S.; Kaji, R.; Goto, S. Bilateral pallidal deep brain stimulation in primary meige syndrome. Parkinsonism Relat. Disord. 2011, 17, 123–125. [Google Scholar] [CrossRef]
- Munts, A.G.; Koehler, P.J. How psychogenic is dystonia? Views from past to present. Brain 2010, 133, 1552–1564. [Google Scholar] [CrossRef] [Green Version]
- Breakefield, X.O.; Blood, A.J.; Li, Y.; Hallett, M.; Hanson, P.I.; Standaert, D.G. The pathophysiological basis of dystonias. Nat. Rev. Neurosci. 2008, 9, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Zurowski, M.; McDonald, W.M.; Fox, S.; Marsh, L. Psychiatric comorbidities in dystonia: Emerging concepts. Mov. Disord. 2013, 28, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S.; Williams, D.T. Psychogenic dystonia. Adv. Neurol. 1988, 50, 431–455. [Google Scholar] [PubMed]
- Tang, J.K.; Moro, E.; Mahant, N.; Hutchison, W.D.; Lang, A.E.; Lozano, A.M.; Dostrovsky, J.O. Neuronal firing rates and patterns in the globus pallidus internus of patients with cervical dystonia differ from those with parkinson’s disease. J. Neurophysiol. 2007, 98, 720–729. [Google Scholar] [CrossRef]
- Hutchison, W.D.; Lang, A.E.; Dostrovsky, J.O.; Lozano, A.M. Pallidal neuronal activity: Implications for models of dystonia. Ann. Neurol. 2003, 53, 480–488. [Google Scholar] [CrossRef]
- Prescott, I.A.; Marino, R.A.; Levy, R. Field evoked potentials in the globus pallidus of non-human primates. Neurosci. Res. 2017, 120, 18–27. [Google Scholar] [CrossRef]
- Nishibayashi, H.; Ogura, M.; Kakishita, K.; Tanaka, S.; Tachibana, Y.; Nambu, A.; Kita, H.; Itakura, T. Cortically evoked responses of human pallidal neurons recorded during stereotactic neurosurgery. Mov. Disord. 2011, 26, 469–476. [Google Scholar] [CrossRef]
- Raz, A.; Vaadia, E.; Bergman, H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J. Neurosci. 2000, 20, 8559–8571. [Google Scholar] [CrossRef]
- Nambu, A.; Chiken, S.; Shashidharan, P.; Nishibayashi, H.; Ogura, M.; Kakishita, K.; Tanaka, S.; Tachibana, Y.; Kita, H.; Itakura, T. Reduced pallidal output causes dystonia. Front. Syst. Neurosci. 2011, 5, 89. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.H.; Chen, T.Y.; Lin, S.Z.; Shyr, M.H.; Chou, Y.C.; Hsieh, W.A.; Tsai, S.T.; Chen, S.Y. Subthalamic deep brain stimulation after anesthetic inhalation in parkinson disease: A preliminary study. J. Neurosurg. 2008, 109, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Tsai, S.T.; Chuang, W.Y.; Kuo, C.C.; Chao, P.C.; Chen, T.Y.; Hung, H.Y.; Chen, S.Y. Dorsolateral subthalamic neuronal activity enhanced by median nerve stimulation characterizes parkinson’s disease during deep brain stimulation with general anesthesia. J. Neurosurg. 2015, 123, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Stahl, C.M.; Frucht, S.J. Focal task specific dystonia: A review and update. J. Neurol. 2017, 264, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Newby, R.; Alty, J.; Kempster, P. Functional dystonia and the borderland between neurology and psychiatry: New concepts. Mov. Disord. 2016, 31, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Ranawaya, R.; Riley, D.; Lang, A. Psychogenic dyskinesias in patients with organic movement disorders. Mov. Disord. 1990, 5, 127–133. [Google Scholar] [CrossRef]
- Tippett, L.J.; Waldvogel, H.J.; Thomas, S.J.; Hogg, V.M.; van Roon-Mom, W.; Synek, B.J.; Graybiel, A.M.; Faull, R.L. Striosomes and mood dysfunction in huntington’s disease. Brain 2007, 130, 206–221. [Google Scholar] [CrossRef] [Green Version]
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Mean ± SD | |
|---|---|---|---|---|---|---|
| Age (yr) at the surgery | 15 | 33 | 35 | 22 | 36 | 28.2 ± 9.3 |
| Sex | F | F | F | F | M | - |
| Age at onset (yr) | 13 | 25 | 32 | 20 | 34 | 24.8 ± 8.6 |
| Duration of disease (yr) | 2 | 8 | 3 | 2 | 1.5 | 3.3 ± 2.7 |
| Type of dystonia | Generalized | Generalized | Generalized | Generalized | Hemidystonia | - |
| Core dystonic feature | a | a, b | a, b | b | a | - |
| Other symptoms related to the organic dystonia | c | c, d | c, e | c, e, f | c, e | - |
| Fahn-Williams criteria | Clinically established | Documented | Documented | Documented | Documented | - |
| Gupta and Lang proposed revisions | Clinically definite | Clinically definite | Clinically definite | Clinically definite | Clinically definite | - |
| Diagnosed psychiatric disturbance | BPD | Panic disorder, DD | DD | BPD | None | - |
| Therapies received before (upper column) and after (lower column) DBS | M, BTX, R, PT | M, BTX, rTMS, R | M, BTX, baclofen (trial), R | M, BTX, R, PT | M, BTX, R | |
| M, BTX, baclofen (trial), R, PT, OSSCS | M, R | M, R | M, R, PT | M, BTX, R |
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Mean ± SD | |
|---|---|---|---|---|---|---|
| Follow-up after surgery (months) | 55 | 52 | 36 | 13 | 9 | 33.0 ± 21.4 |
| Implanted devices system | Model 3387 Medtronic Activa SC | 4 contacts leads Abbott Brio | 4 contacts leads Abbott Brio | Directional leads BS Vercise Gevia | Directional lead BS Vercise Gevia | - |
| Stimulation parameters | ||||||
| Electrode right/left | 2+1-/2+1- bipolar | 2+1-/2+1- bipolar | C+2-/C+2- monopolar | C+1-2-3-4-/C+1-2-3-4- monopolar | NA/C+3-4-5-6- monopolar | - |
| Current (mA) right/left | 1.5/3.1 | 1.1/1.6 | 2.3/1.9 | 4.6/4.6 | -/4.8 | 2.2 ± 1.6/3.2 ± 1.5 |
| Pulse width (μS) right/left | 120/420 | 200/250 | 200/150 | 60/60 | /180 | 110 ± 66.3/194 ± 150.3 |
| Frequency (Hz) right/left | 80/80 | 60/60 | 180/180 | 130/130 | /20 | 100 ± 35.6/84 ± 47.2 |
| BFMDRS-M (max = 120) | ||||||
| Preoperatively | 70 | 68.5 | 59.5 | 51 | 45.5 | 58.9 ± 10.7 |
| Postoperatively | 26 | 0 | 1 | 1 | 21 | 9.8 ± 12.6 |
| Percent improvement (%) | 62.9 | 100 | 98.3 | 98.0 | 53.8 | 82.6 ± 22.4 |
| BFMDRS-D (max = 30) | ||||||
| Preoperatively | 14 | 16 | 13 | 10 | 11 | 12.8 ± 2.4 |
| Postoperatively | 7 | 0 | 0 | 0 | 9 | 3.2 ± 4.4 |
| Percent improvement (%) | 50.0 | 100 | 100 | 100 | 18.2 | 73.6 ± 37.8 |
| Type of Dystonia | Age (yr) | Duration (yr) | BFMDRS-M (max = 120), Mean ± S.D. | BFMDRS-D (max = 30) Mean ± S.D. | ||||
|---|---|---|---|---|---|---|---|---|
| Preoperatively | Postoperatively | Percent Improvement (%) | Preoperatively | Postoperatively | Percent Improvement (%) | |||
| Functional dystonia (n = 5) | 28.2 ± 9.3 *,†† | 3.3 ± 2.7 †† | 58.9 ± 10.7 † | 9.8 ± 12.6 | 82.6 ± 22.4 | 12.8 ± 2.4 | 3.2 ± 4.4 | 73.6 ± 37.8 |
| Tardive dystonia [30] (n = 6) | 44.5 ± 8.7 | 3.1 ± 2.2 †† | 30.8 ± 22.7 | 3.8 ± 3.8 | 85.5 ± 14.4 | 9.3 ± 4.8 | 1.8 ± 1.7 | 80.2 ± 12.2 |
| Meige syndrome [31] (n = 5) | 64.6 ± 7.2 | 12.4 ± 4.2 | 22.2 ± 12.4 | 3.1 ± 1.7 | 84.2 ± 6.8 | 11.2 ± 7.9 | 1.4 ± 1.5 | 89.4 ± 8.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morigaki, R.; Miyamoto, R.; Mure, H.; Fujita, K.; Matsuda, T.; Yamamoto, Y.; Nakataki, M.; Okahisa, T.; Matsumoto, Y.; Miyake, K.; et al. Can Pallidal Deep Brain Stimulation Rescue Borderline Dystonia? Possible Coexistence of Functional (Psychogenic) and Organic Components. Brain Sci. 2020, 10, 636. https://doi.org/10.3390/brainsci10090636
Morigaki R, Miyamoto R, Mure H, Fujita K, Matsuda T, Yamamoto Y, Nakataki M, Okahisa T, Matsumoto Y, Miyake K, et al. Can Pallidal Deep Brain Stimulation Rescue Borderline Dystonia? Possible Coexistence of Functional (Psychogenic) and Organic Components. Brain Sciences. 2020; 10(9):636. https://doi.org/10.3390/brainsci10090636
Chicago/Turabian StyleMorigaki, Ryoma, Ryosuke Miyamoto, Hideo Mure, Koji Fujita, Taku Matsuda, Yoko Yamamoto, Masahito Nakataki, Tetsuya Okahisa, Yuki Matsumoto, Kazuhisa Miyake, and et al. 2020. "Can Pallidal Deep Brain Stimulation Rescue Borderline Dystonia? Possible Coexistence of Functional (Psychogenic) and Organic Components" Brain Sciences 10, no. 9: 636. https://doi.org/10.3390/brainsci10090636
APA StyleMorigaki, R., Miyamoto, R., Mure, H., Fujita, K., Matsuda, T., Yamamoto, Y., Nakataki, M., Okahisa, T., Matsumoto, Y., Miyake, K., Yamamoto, N., Kaji, R., Takagi, Y., & Goto, S. (2020). Can Pallidal Deep Brain Stimulation Rescue Borderline Dystonia? Possible Coexistence of Functional (Psychogenic) and Organic Components. Brain Sciences, 10(9), 636. https://doi.org/10.3390/brainsci10090636