Genotype- and Age-Dependent Differences in Ultrasound Vocalizations of SPRED2 Mutant Mice Revealed by Machine Deep Learning
Abstract
:1. Introduction
2. Materials and Methods
2.1. SPRED2 Mutant Mice
2.2. Recording and Hardware
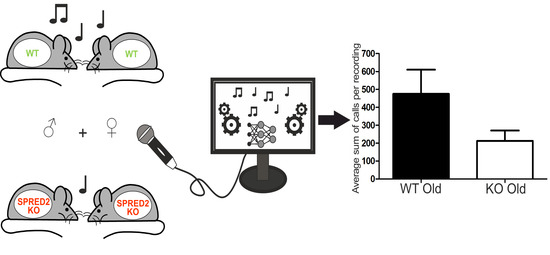
2.3. Experimental Setup “Speed Dating”
2.4. Call Analysis and Classification
2.5. Statistics
3. Results
3.1. Genotype- and Age-Dependent Call Classification
3.2. Genotype-Dependent Call Syntax Differences
3.3. Genotype- and Age-Dependent Differences in Call Characteristics
3.4. T-SNE Plot Visualization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Leong, K.M.; Ortolani, A.; Graham, L.H.; Savage, A. The use of low-frequency vocalizations in African elephant (Loxodonta africana) reproductive strategies. Horm. Behav. 2003, 43, 433–443. [Google Scholar] [CrossRef]
- Reby, D.; Charlton, B.D.; Locatelli, Y.; McComb, K. Oestrous red deer hinds prefer male roars with higher fundamental frequencies. Proc. Biol. Sci. 2010, 277, 2747–2753. [Google Scholar] [CrossRef] [Green Version]
- Doupe, A.J.; Patricia, K.K. Birdsong and human speech: Common themes and mechanisms. Annu. Rev. Neurosci. 1999, 22, 567–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holy, T.E.; Zhongsheng, G. Ultrasonic songs of male mice. PLoS Biol. 2005, 3, e386. [Google Scholar] [CrossRef]
- Liu, R.C.; Miller, K.D. Acoustic variability and distinguishability among mouse ultrasound vocalizations. J. Acoust. Soc. Am. 2003, 114, 3412–3422. [Google Scholar] [CrossRef] [PubMed]
- Whitney, G.; Coble, J.R.; Stockton, M.D.; Tilson, E.F. Ultrasonic emissions: Do they facilitate courtship of mice. J. Comp. Physiol. Psychol. 1973, 84, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Warburton, V.L.; Sales, G.D.; Milligan, S.R. The emission and elicitation of mouse ultrasonic vocalizations: The effects of age, sex and gonadal status. Physiol. Behav. 1989, 45, 41–47. [Google Scholar] [CrossRef]
- Maggio, J.C.; Whitney, G. Ultrasonic vocalizing by adult female mice (Mus musculus). J. Comp. Psychol. 1985, 99, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Moles, A.; Costantini, F.; Garbugino, L.; Zanettini, C.; D’Amato, F.R. Ultrasonic vocalizations emitted during dyadic interactions in female mice: A possible index of sociability? Behav. Brain Res. 2007, 182, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Neunuebel, J.P.; Taylor, A.L.; Arthur, B.J.; Egnor, S.R. Female mice ultrasonically interact with males during courtship displays. Elife 2015, 4, e06203. [Google Scholar] [CrossRef]
- Pomerantz, S.M.; Nunez, A.A.; Bean, N.J. Female behavior is affected by male ultrasonic vocalizations in house mice. Physiol. Behav. 1983, 31, 91–96. [Google Scholar] [CrossRef]
- Hanson, J.L.; Hurley, L.M. Female presence and estrous state influence mouse ultrasonic courtship vocalizations. PLoS ONE 2012, 7, e40782. [Google Scholar] [CrossRef] [Green Version]
- Chabout, J.; Sarkar, A.; Dunson, D.B.; Jarvis, E.D. Male mice song syntax depends on social contexts and influences female preferences. Front. Behav. Neurosci. 2015, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.M.; Gourdon, J.C.; Clarke, P.B. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: Effects of amphetamine and social context. Psychopharmacology 2010, 211, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Scattoni, M.L.; Ricceri, L.; Crawley, J.N. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011, 10, 44–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgdorf, J.; Panksepp, J.; Brudzynski, S.M.; Beinfeld, M.C.; Cromwell, H.C.; Kroes, R.A.; Moskal, J.R. The effects of selective breeding for differential rates of 50-kHz ultrasonic vocalizations on emotional behavior in rats. J. Int. Soc. Dev. Psychobiol. 2009, 51, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Jelen, P.; Soltysik, S.; Zagrodzka, J. 22-kHz ultrasonic vocalization in rats as an index of anxiety but not fear: Behavioral and pharmacological modulation of affective state. Behav. Brain Res. 2003, 141, 63–72. [Google Scholar] [CrossRef]
- Panksepp, J.B.; Jochman, K.A.; Kim, J.U.; Koy, J.J.; Wilson, E.D.; Chen, Q.; Wilson, C.R.; Lahvis, G.P. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS ONE 2007, 2, e351. [Google Scholar] [CrossRef]
- Sugimoto, H.; Okabe, S.; Kato, M.; Koshida, N.; Shiroishi, T.; Mogi, K.; Kikusui, T.; Koide, T. A role for strain differences in waveforms of ultrasonic vocalizations during male-female interaction. PLoS ONE 2011, 6, e22093. [Google Scholar] [CrossRef] [Green Version]
- White, N.R.; Prasad, M.; Barfield, R.J.; Nyby, J.G. 40- and 70-kHz vocalizations of mice (Mus musculus) during copulation. Physiol. Behav. 1998, 63, 467–473. [Google Scholar] [CrossRef]
- Ullrich, M.; Bundschu, K.; Benz, P.M.; Abesser, M.; Freudinger, R.; Fischer, T.; Ullrich, J.; Renné, T.; Walter, U.; Schuh, K. Identification of SPRED2 (sprouty-related protein with EVH1 domain 2) as a negative regulator of the hypothalamic-pituitary-adrenal axis. J. Biol. Chem. 2011, 286, 9477–9488. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, M.; Weber, M.; Post, A.M.; Popp, S.; Grein, J.; Zechner, M.; González, H.G.; Kreis, A.; Schmitt, A.G.; Üçeyler, N.; et al. OCD-like behavior is caused by dysfunction of thalamo-amygdala circuits and upregulated TrkB/ERK-MAPK signaling as a result of SPRED2 deficiency. Mol. Psychiatry 2018, 23, 444–458. [Google Scholar] [CrossRef]
- Sapir, S. Multiple factors are involved in the dysarthria associated with Parkinson’s disease: A review with implications for clinical practice and research. J. Speech Lang. Hear. Res. 2014, 57, 1330–1343. [Google Scholar] [CrossRef] [PubMed]
- Coffey, K.R.; Marx, R.G.; Neumaier, J.F. DeepSqueak: A deep learning-based system for detection and analysis of ultrasonic vocalizations. Neuropsychopharmacology 2019, 44, 859–868. [Google Scholar] [CrossRef] [Green Version]
- Bundschu, K.; Knobeloch, K.-P.; Ullrich, M.; Schinke, T.; Amling, M.; Engelhardt, C.M.; Renné, T.; Walter, U.; Schuh, K. Gene disruption of Spred-2 causes dwarfism. J. Biol. Chem. 2005, 280, 28572–28580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Champlin, A.K.; Dorr, D.L.; Gates, A.H. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol. Reprod. 1973, 8, 491–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; Taft, R.A. Mouse estrous cycle identification tool and images. PLoS ONE 2012, 7, e35538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, A.H.; Santana, G.M.; Ortiz GM, B.; Bampi, S.; Dietrich, M.O. Analysis of ultrasonic vocalizations from mice using computer vision and machine learning. Elife 2021, 10, e59161. [Google Scholar] [CrossRef]
- Scattoni, M.L.; Gandhy, S.U.; Ricceri, L.; Crawley, J.N. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS ONE 2008, 3, e3067. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liang, S.; Burgdorf, J.; Wess, J.; Yeomans, J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS ONE 2008, 3, e1893. [Google Scholar]
- Zheng, Y.; Zhiyi, Z. Learning and memory dysfunction of non-surgery cage-mates of mice with surgery. Stress 2020, 23, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Arriaga, G.; Jarvis, E.D. Mouse vocal communication system: Are ultrasounds learned or innate? Brain Lang. 2013, 124, 96–116. [Google Scholar] [CrossRef] [PubMed]
- Brems, H.; Pasmant, E.; Van Minkelen, R.; Wimmer, K.; Upadhyaya, M.; Legius, E.; Messiaen, L. Review and update of SPRED1 mutations causing Legius syndrome. Hum. Mutat. 2012, 33, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Denayer, E.; Ahmed, T.; Brems, H.; Van Woerden, G.; Borgesius, N.Z.; Callaerts-Vegh, Z.; Yoshimura, A.; Hartmann, D.; Elgersma, Y.; D’Hooge, R.; et al. Spred1 is required for synaptic plasticity and hippocampus-dependent learning. J. Neurosci. 2008, 28, 14443–14449. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Short | duration of less than 10 ms |
| Flat | longer than 10 ms but no large change in delta frequency |
| Up | monotonically increasing in frequency |
| Down | monotonically decreasing in frequency |
| U | U-shaped calls, frequency drops in the beginning but then rises again to about the same as it started |
| Inverted U | inverted version of the U call |
| Step Down | two calls which closely follow each other and have a frequency change to a lower frequency |
| Step Up | two calls which closely follow each other and have frequency a change to a higher frequency |
| Frequency Steps | long calls (>50 ms) with instantaneous frequency changes appearing as stepsbut with no interruption in time |
| Complex | long calls (>50 ms) with a straight course and variation in frequencyor calls with two different parallel frequencies at the same time (harmonic component) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hepbasli, D.; Gredy, S.; Ullrich, M.; Reigl, A.; Abeßer, M.; Raabe, T.; Schuh, K. Genotype- and Age-Dependent Differences in Ultrasound Vocalizations of SPRED2 Mutant Mice Revealed by Machine Deep Learning. Brain Sci. 2021, 11, 1365. https://doi.org/10.3390/brainsci11101365
Hepbasli D, Gredy S, Ullrich M, Reigl A, Abeßer M, Raabe T, Schuh K. Genotype- and Age-Dependent Differences in Ultrasound Vocalizations of SPRED2 Mutant Mice Revealed by Machine Deep Learning. Brain Sciences. 2021; 11(10):1365. https://doi.org/10.3390/brainsci11101365
Chicago/Turabian StyleHepbasli, Denis, Sina Gredy, Melanie Ullrich, Amelie Reigl, Marco Abeßer, Thomas Raabe, and Kai Schuh. 2021. "Genotype- and Age-Dependent Differences in Ultrasound Vocalizations of SPRED2 Mutant Mice Revealed by Machine Deep Learning" Brain Sciences 11, no. 10: 1365. https://doi.org/10.3390/brainsci11101365
APA StyleHepbasli, D., Gredy, S., Ullrich, M., Reigl, A., Abeßer, M., Raabe, T., & Schuh, K. (2021). Genotype- and Age-Dependent Differences in Ultrasound Vocalizations of SPRED2 Mutant Mice Revealed by Machine Deep Learning. Brain Sciences, 11(10), 1365. https://doi.org/10.3390/brainsci11101365