Modulation of Interhemispheric Inhibition between Primary Motor Cortices Induced by Manual Motor Imitation: A Transcranial Magnetic Stimulation Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.2.1. General Design
2.2.2. Preparation
2.2.3. EMG Feedback Alternation
2.2.4. Main Experiment
2.2.5. Plastic Change Analysis
2.2.6. EMG Recording
2.2.7. TMS Parameters
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
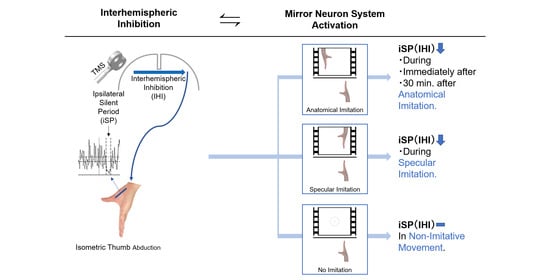
3.1. iSP Modulation during Different Imitation Conditions
3.2. Chronological Plastic Changes of iSP
4. Discussion
4.1. M1–M1 IHI Modulation during Imitation
4.2. Hand Dominance Did Not Affect IHI during Imitation
4.3. Plastic Effects Differ between Imitation Patterns
4.4. Significance and Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hellige, J.B. Interhemispheric Interaction: Models, Paradigms and Recent Findings. In Duality and Unity of the Brain; Springer: Boston, MA, USA, 1987; pp. 454–465. [Google Scholar]
- Daffertshofer, A.; Peper, C.L.E.; Beek, P.J. Stabilization of bimanual coordination due to active interhemispheric inhibition: A dynamical account. Biol. Cybern. 2005, 92, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Carson, R.G. Neural pathways mediating bilateral interactions between the upper limbs. Brain Res. Rev. 2005, 49, 641–662. [Google Scholar] [CrossRef]
- Marzi, C.A. The Poffenberger paradigm: A first, simple, behavioural tool to study interhemispheric transmission in humans. Brain Res. Bull. 1999, 50, 421–422. [Google Scholar] [CrossRef]
- Braun, C.M.J.; Collin, I.; Mailloux, C. The “Poffenberger” and “Dimond” paradigms: Interrelated approaches to the study of interhemispheric dynamics? Brain Cogn. 1997, 34, 337–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bütefisch, C.M.; Weβling, M.; Netz, J.; Seitz, R.J.; Hömberg, V. Relationship Between Interhemispheric Inhibition and Motor Cortex Excitability in Subacute Stroke Patients. Neurorehabilit. Neural Repair 2008, 22, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Fiori, F.; Chiappini, E.; Candidi, M.; Romei, V.; Borgomaneri, S.; Avenanti, A. Long-latency interhemispheric interactions between motor-related areas and the primary motor cortex: A dual site TMS study. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duque, J.; Hummel, F.; Celnik, P.; Murase, N.; Mazzocchio, R.; Cohen, L.G. Transcallosal inhibition in chronic subcortical stroke. NeuroImage 2005, 28, 940–946. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.N.; Stinear, C.M. TMS measures of motor cortex function after stroke: A meta-analysis. Brain Stimul. 2017, 10, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Ferbert, A.; Priori, A.; Rothwell, J.C.; Day, B.L.; Colebatch, J.G.; Marsden, C.D. Interhemispheric inhibition of the human motor cortex. J. Physiol. 1992, 453, 525–546. [Google Scholar] [CrossRef] [PubMed]
- Hanajima, R.; Ugawa, Y.; Machii, K.; Mochizuki, H.; Terao, Y.; Enomoto, H.; Furubayashi, T.; Shiio, Y.; Uesugi, H.; Kanazawa, I. Interhemispheric facilitation of the hand motor area in humans. J. Physiol. 2001, 531 Pt 3, 849–859. [Google Scholar] [CrossRef]
- Meyer, B.-U.; Röricht, S.; Von Einsiedel, H.G.; Kruggel, F.; Weindl, A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain 1995, 118, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.A.; Cohen, L.G. Interhemispheric inhibition between primary motor cortices: What have we learned? J. Physiol. 2009, 587 Pt 4, 725–726. [Google Scholar] [CrossRef]
- Avanzino, L.; Teo, J.T.H.; Rothwell, J.C. Intracortical circuits modulate transcallosal inhibition in humans. J. Physiol. 2007, 583, 99–114. [Google Scholar] [CrossRef]
- Beaulé, V.; Tremblay, S.; Théoret, H. Interhemispheric Control of Unilateral Movement. Neural Plast. 2012, 2012, 627816. [Google Scholar] [CrossRef] [PubMed]
- Vercauteren, K.; Pleysier, T.; Van Belle, L.; Swinnen, S.P.; Wenderoth, N. Unimanual muscle activation increases interhemispheric inhibition from the active to the resting hemisphere. Neurosci. Lett. 2008, 445, 209–213. [Google Scholar] [CrossRef]
- Giovannelli, F.; Borgheresi, A.; Balestrieri, F.; Zaccara, G.; Viggiano, M.P.; Cincotta, M.; Ziemann, U. Modulation of interhemispheric inhibition by volitional motor activity: An ipsilateral silent period study. J. Physiol. 2009, 587, 5393–5410. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J.; Dettmers, C.; Terborg, C.; Weiller, C. Inhibition of ipsilateral motor cortex during phasic generation of low force. Clin. Neurophysiol. 2001, 112, 114–121. [Google Scholar] [CrossRef]
- Reid, C.S.; Serrien, D.J. Handedness and the excitability of cortical inhibitory circuits. Behav. Brain Res. 2012, 230, 144–148. [Google Scholar] [CrossRef]
- Duque, J.; Mazzocchio, R.; Dambrosia, J.; Murase, N.; Olivier, E.; Cohen, L. Kinematically Specific Interhemispheric Inhibition Operating in the Process of Generation of a Voluntary Movement. Cereb. Cortex 2005, 15, 588–593. [Google Scholar] [CrossRef]
- Murase, N.; Duque, J.; Mazzocchio, R.; Cohen, L.G. Influence of interhemispheric interactions on motor function in chronic stroke. Ann. Neurol. 2004, 55, 400–409. [Google Scholar] [CrossRef]
- Kirton, A.; DeVeber, G.; Gunraj, C.; Chen, R. Cortical excitability and interhemispheric inhibition after subcortical pediatric stroke: Plastic organization and effects of rTMS. Clin. Neurophysiol. 2010, 121, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.A.; Machado, A.; Janini, D.; Varnerin, N.; Bonnett, C.; Yue, G.; Jones, S.; Lowe, M.; Beall, E.; Sakaie, K.; et al. Assessment of Inter-Hemispheric Imbalance Using Imaging and Noninvasive Brain Stimulation in Patients With Chronic Stroke. Arch. Phys. Med. Rehabil. 2015, 96, S94–S103. [Google Scholar] [CrossRef] [Green Version]
- Liepert, J.; Hamzei, F.; Weiller, C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve 2000, 23, 1761–1763. [Google Scholar] [CrossRef]
- Ward, N.S.; Cohen, L.G. Mechanisms Underlying Recovery of Motor Function After Stroke. Arch. Neurol. 2004, 61, 1844–1848. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Branscheidt, M.; Schambra, H.; Steiner, L.; Widmer, M.; Diedrichsen, J.; Goldsmith, J.; Lindquist, M.; Kitago, T.; Luft, A.R.; et al. Rethinking interhemispheric imbalance as a target for stroke neurorehabilitation. Ann. Neurol. 2019, 85, 502–513. [Google Scholar] [CrossRef] [Green Version]
- Di Pino, G.; Pellegrino, G.; Assenza, G.; Capone, F.; Ferreri, F.; Formica, D.; Ranieri, F.; Tombini, M.; Ziemann, U.; Rothwell, J.C. Modulation of brain plasticity in stroke: A novel model for neurorehabilitation. Nat. Rev. Neurol. 2014, 10, 597–608. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Potter-Baker, K.A.; Cunningham, D.A.; Li, M.; Sankarasubramanian, V.; Lee, J.; Jones, S.; Sakaie, K.; Wang, X.; Machado, A.G.; et al. Stratifying chronic stroke patients based on the influence of contralesional motor cortices: An inter-hemispheric inhibition study. Clin. Neurophysiol. 2020, 131, 2516–2525. [Google Scholar] [CrossRef]
- Ertelt, D.; Small, S.; Solodkin, A.; Dettmers, C.; McNamara, A.; Binkofski, F.; Buccino, G. Action observation has a positive impact on rehabilitation of motor deficits after stroke. NeuroImage 2007, 36, T164–T173. [Google Scholar] [CrossRef] [PubMed]
- Celnik, P.; Webster, B.; Glasser, D.M.; Cohen, L.G. Effects of Action Observation on Physical Training After Stroke. Stroke 2008, 39, 1814–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschini, M.; Ceravolo, M.G.; Agosti, M.; Cavallini, P.; Bonassi, S.; Dall’Armi, V.; Massucci, M.; Schifini, F.; Sale, P. Clinical relevance of action observation in upper-limb stroke rehabilitation: A possible role in recovery of functional dexterity. A randomized clinical trial. Neurorehabilit. Neural Repair 2012, 26, 456–462. [Google Scholar] [CrossRef]
- Zhu, J.-D.; Cheng, C.-H.; Tseng, Y.-J.; Chou, C.-C.; Chen, C.-C.; Hsieh, Y.-W.; Liao, Y.-H. Modulation of Motor Cortical Activities by Action Observation and Execution in Patients with Stroke: An MEG Study. Neural Plast. 2019, 2019, 8481371. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Craighero, L. THE MIRROR-NEURON SYSTEM. Annu. Rev. Neurosci. 2004, 27, 169–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grezes, J.; Decety, J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: A meta-analysis. Human Brain Mapp. 2001, 12, 1–19. [Google Scholar] [CrossRef]
- Di Pellegrino, G.; Fadiga, L.; Fogassi, L.; Gallese, V.; Rizzolatti, G. Understanding motor events: A neurophysiological study. Exp. Brain Res. 1992, 91, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Gallese, V.; Fadiga, L.; Fogassi, L.; Rizzolatti, G. Action recognition in the premotor cortex. Brain 2009, 132, 1685–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cattaneo, L.; Rizzolatti, G. The Mirror Neuron System. Arch. Neurol. 2009, 66, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Chong, T.T.-J.; Cunnington, R.; Williams, M.A.; Kanwisher, N.; Mattingley, J.B. fMRI Adaptation Reveals Mirror Neurons in Human Inferior Parietal Cortex. Curr. Biol. 2008, 18, 1576–1580. [Google Scholar] [CrossRef] [Green Version]
- Buccino, G.; Binkofski, F.; Riggio, L. The mirror neuron system and action recognition. Brain Lang. 2004, 89, 370–376. [Google Scholar] [CrossRef]
- Zhang, J.J.Q.; Fong, K.N.K.; Welage, N.; Liu, K.P.Y. The Activation of the Mirror Neuron System during Action Observation and Action Execution with Mirror Visual Feedback in Stroke: A Systematic Review. Neural Plast. 2018, 2018, 2321045. [Google Scholar] [CrossRef] [PubMed]
- Krams, M.; Rushworth, M.F.S.; Deiber, M.-P.; Frackowiak, R.S.J.; Passingham, R.E. The preparation, execution and suppression of copied movements in the human brain. Exp. Brain Res. 1998, 120, 386–398. [Google Scholar] [CrossRef]
- Frenkel-Toledo, S.; Liebermann, D.G.; Bentin, S.; Soroker, N. Dysfunction of the Human Mirror Neuron System in Ideomotor Apraxia: Evidence from Mu Suppression. J. Cogn. Neurosci. 2016, 28, 775–791. [Google Scholar] [CrossRef]
- Iacoboni, M. Imitation, Empathy, and Mirror Neurons. Annu. Rev. Psychol. 2009, 60, 653–670. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Ye, Q.; Ji, X.; Zhang, S.; Yang, X.; Zhou, Q.; Cong, F.; Chen, W.; Zhang, X.; Zhang, B.; et al. Mirror neuron system based therapy for aphasia rehabilitation. Front. Psychol. 2015, 6, 1665. [Google Scholar] [CrossRef] [Green Version]
- Perry, A.; Saunders, S.N.; Stiso, J.; Dewar, C.; Lubell, J.; Meling, T.R.; Solbakk, A.-K.; Endestad, T.; Knight, R.T. Effects of prefrontal cortex damage on emotion understanding: EEG and behavioural evidence. Brain 2017, 140, 1086–1099. [Google Scholar] [CrossRef]
- Macuga, K.L.; Frey, S.H. Neural representations involved in observed, imagined, and imitated actions are dissociable and hierarchically organized. NeuroImage 2012, 59, 2798–2807. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, R.; Watanabe, S.; Kuruma, H.; Murakami, Y.; Seno, A.; Matsuda, T. Neural activation during imitation of movements presented from four different perspectives: A functional magnetic resonance imaging study. Neurosci. Lett. 2011, 503, 100–104. [Google Scholar] [CrossRef]
- Bekkering, H.; Wohlschlager, A.; Gattis, M. Imitation of gestures in children is goal-directed. Q. J. Exp. Psychol. Sect. A 2000, 53, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Koski, L.; Iacoboni, M.; Dubeau, M.-C.; Woods, R.P.; Mazziotta, J.C.; Kann, O.; Kovacs, R.; Heinemann, U. Modulation of Cortical Activity During Different Imitative Behaviors. J. Neurophysiol. 2003, 89, 460–471. [Google Scholar] [CrossRef]
- Miyamoto, R.; Kikuchi, Y.; Senoo, A. Distinctive neural basis of anatomic imitation compared with specular imitation. J. J. Jpn. Acad. Health Sci. 2008, 11, 153–161. [Google Scholar] [CrossRef]
- Mengotti, P.; Corradi-Dell’Acqua, C.; Rumiati, R.I. Imitation components in the human brain: An fMRI study. NeuroImage 2012, 59, 1622–1630. [Google Scholar] [CrossRef]
- Aziz-Zadeh, L.; Koski, L.; Zaidel, E.; Mazziotta, J.; Iacoboni, M. Lateralization of the Human Mirror Neuron System. J. Neurosci. 2006, 26, 2964–2970. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Sinigaglia, C. The functional role of the parieto-frontal mirror circuit: Interpretations and misinterpretations. Nat. Rev. Neurosci. 2010, 11, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Saleh, S.; Yarossi, M.; Manuweera, T.; Adamovich, S.; Tunik, E. Network interactions underlying mirror feedback in stroke: A dynamic causal modeling study. NeuroImage Clin. 2017, 13, 46–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avanzino, L.; Raffo, A.; Pelosin, E.; Ogliastro, C.; Marchese, R.; Ruggeri, P.; Abbruzzese, G. Training based on mirror visual feedback influences transcallosal communication. Eur. J. Neurosci. 2014, 40, 2581–2588. [Google Scholar] [CrossRef]
- Läppchen, C.; Ringer, T.; Blessin, J.; Schulz, K.; Seidel, G.; Lange, R.; Hamzei, F. Daily iTBS worsens hand motor training—A combined TMS, fMRI and mirror training study. NeuroImage 2015, 107, 257–265. [Google Scholar] [CrossRef]
- Nojima, I.; Mima, T.; Koganemaru, S.; Thabit, M.N.; Fukuyama, H.; Kawamata, T. Human Motor Plasticity Induced by Mirror Visual Feedback. J. Neurosci. 2012, 32, 1293–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, N.J.; van Koningsbruggen, M. “Non-invasive” brain stimulation is not non-invasive. Front. Syst. Neurosci. 2013, 7, 76. [Google Scholar] [CrossRef]
- Rossi, S.; Antal, A.; Bestmann, S.; Bikson, M.; Brewer, C.; Brockmöller, J.; Carpenter, L.L.; Cincotta, M.; Chen, R.; Daskalakis, J.D.; et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clin. Neurophysiol. 2020, 132, 269–306. [Google Scholar] [CrossRef]
- Oldfield, R. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Keel, J.C.; Smith, M.J.; Wassermann, E.M. A safety screening questionnaire for transcranial magnetic stimulation. Clin. Neurophysiol. 2001, 112, 720. [Google Scholar] [CrossRef]
- Jung, P.; Ziemann, U. Differences of the ipsilateral silent period in small hand muscles. Muscle Nerve 2006, 34, 431–436. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Dubuc, T.; Boufadel, D.F.; Fisher, B.E. Measuring ipsilateral silent period: Effects of muscle contraction levels and quantification methods. Brain Res. 2017, 1674, 77–83. [Google Scholar] [CrossRef]
- Baweja, H.S.; Patel, B.K.; Martinkewiz, J.D.; Vu, J.; Christou, E.A. Removal of visual feedback alters muscle activity and reduces force variability during constant isometric contractions. Exp. Brain Res. 2009, 197, 35–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossini, P.; Burke, D.; Chen, R.; Cohen, L.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.; George, M.; et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef]
- Chen, R.; Cros, D.; Curra, A.; Di Lazzaro, V.; Lefaucheur, J.-P.; Magistris, M.R.; Mills, K.; Rösler, K.M.; Triggs, W.J.; Ugawa, Y.; et al. The clinical diagnostic utility of transcranial magnetic stimulation: Report of an IFCN committee. Clin. Neurophysiol. 2008, 119, 504–532. [Google Scholar] [CrossRef]
- Ziemann, U.; Hallett, M. Hemispheric asymmetry of ipsilateral motor cortex activation during unimanual motor tasks: Further evidence for motor dominance. Clin. Neurophysiol. 2001, 112, 107–113. [Google Scholar] [CrossRef]
- Stinear, C.M.; Petoe, M.A.; Byblow, W.D. Primary Motor Cortex Excitability During Recovery After Stroke: Implications for Neuromodulation. Brain Stimul. 2015, 8, 1183–1190. [Google Scholar] [CrossRef]
- Davidson, T.; Tremblay, F. Age and hemispheric differences in transcallosal inhibition between motor cortices: An ispsilateral silent period study. BMC Neurosci. 2013, 14, 62. [Google Scholar] [CrossRef] [Green Version]
- Fleming, M.K.; Newham, D.J. Reliability of Transcallosal Inhibition in Healthy Adults. Front. Hum. Neurosci. 2017, 10, 681. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, N.; Tada, T.; Matsuo, Y.; Ikoma, K. Low-Frequency Repetitive TMS Plus Anodal Transcranial DCS Prevents Transient Decline in Bimanual Movement Induced by Contralesional Inhibitory rTMS After Stroke. Neurorehabilit. Neural Repair 2012, 26, 988–998. [Google Scholar] [CrossRef]
- Kessler, K.; Biermann-Ruben, K.; Jonas, M.; Siebner, H.R.; Bäumer, T.; Münchau, A.; Schnitzler, A. Investigating the human mirror neuron system by means of cortical synchronization during the imitation of biological movements. NeuroImage 2006, 33, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.; Dovern, A.; Hesse, M.D.; Ebke, M.; Karbe, H.; Saliger, J.; Fink, G.R.; Weiss-Blankenhorn, P. Lesion evidence for a human mirror neuron system. Cortex 2017, 90, 125–137. [Google Scholar] [CrossRef]
- Gatti, R.; Rocca, M.A.; Fumagalli, S.; Cattrysse, E.; Kerckhofs, E.; Falini, A.; Filippi, M. The effect of action observation/execution on mirror neuron system recruitment: An fMRI study in healthy individuals. Brain Imaging Behav. 2017, 11, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Iacoboni, M. Understanding Others: Imitation, Language, Empathy. In Perspectives on Imitation: From Cognitive Neuroscience to Social Science; Hurley, S., Chater, N., Eds.; MIT Press: Cambridge, MA, USA, 2005; Volume 1, pp. 77–99. [Google Scholar]
- Franz, E.A.; Ford, S.; Werner, S. Brain and cognitive processes of imitation in bimanual situations: Making inferences about mirror neuron systems. Brain Res. 2007, 1145, 138–149. [Google Scholar] [CrossRef]
- Wapner, S.; Cirillo, L. Imitation of a model’s hand movements: Age changes in transposition of left-right relations. Child Dev. 1968, 39, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Pierpaoli, C.; Ferrante, L.; Manzoni, T.; Fabri, M. Anatomical or mirror mode imitation? A behavioral approach. Arch Ital Biol 2014, 152, 20. [Google Scholar]
- Hübers, A.; Orekhov, Y.; Ziemann, U.; Hbers, A. Interhemispheric motor inhibition: Its role in controlling electromyographic mirror activity. Eur. J. Neurosci. 2008, 28, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Fling, B.W.; Seidler, R.D. Task-dependent effects of interhemispheric inhibition on motor control. Behav. Brain Res. 2012, 226, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Pierpaoli, C.; Foschi, N.; Cagnetti, C.; Ferrante, L.; Manzoni, T.; Polonara, G.; Fabri, M. Imitation Strategies in Callosotomized Patients. Arch. Ital. Biol. 2018, 156, 12–26. [Google Scholar] [CrossRef]
- Netz, J.; Ziemann, U.; Hömberg, V. Hemispheric asymmetry of transcallosal inhibition in man. Exp. Brain Res. 1995, 104, 527–533. [Google Scholar] [CrossRef]
- Duque, J.; Murase, N.; Celnik, P.; Hummel, F.; Harris-Love, M.; Mazzocchio, R.; Olivier, E.; Cohen, L.G. Intermanual Differences in Movement-related Interhemispheric Inhibition. J. Cogn. Neurosci. 2007, 19, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Moulton, E.; Galléa, C.; Kemlin, C.; Valabregue, R.; Maier, M.A.; Lindberg, P.; Rosso, C. Cerebello-Cortical Differences in Effective Connectivity of the Dominant and Non-dominant Hand during a Visuomotor Paradigm of Grip Force Control. Front. Hum. Neurosci. 2017, 11, 511. [Google Scholar] [CrossRef]
- Kirby, K.M.; Pillai, S.R.; Carmichael, O.T.; Van Gemmert, A.W.A. Brain functional differences in visuo-motor task adaptation between dominant and non-dominant hand training. Exp. Brain Res. 2019, 237, 3109–3121. [Google Scholar] [CrossRef]
- Malihe Moones, T.; Emami, T.; Hoseini, S.M. The Effect of Initial Practice with Dominant and Non-Dominant Hand on Acquisition, Retention and Transfer of A Complex Motor Task. Biosci. Biotechnol. Res. Asia 2017, 14, 1067. [Google Scholar]
- Nelson, A.J.; Hoque, T.; Gunraj, C.; Ni, Z.; Chen, R. Bi-directional interhemispheric inhibition during unimanual sustained contractions. BMC Neurosci. 2009, 10, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grefkes, C.; Eickhoff, S.B.; Nowak, D.A.; Dafotakis, M.; Fink, G.R. Dynamic intra- and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. NeuroImage 2008, 41, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Casula, E.P.; Maiella, M.; Pellicciari, M.C.; Porrazzini, F.; D’Acunto, A.; Rocchi, L.; Koch, G. Novel TMS-EEG indexes to investigate interhemispheric dynamics in humans. Clin. Neurophysiol. 2020, 131, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Casula, E.P.; Pellicciari, M.C.; Bonnì, S.; Spanò, B.; Ponzo, V.; Salsano, I.; Giulietti, G.; Cinnera, A.M.; Maiella, M.; Borghi, I.; et al. Evidence for interhemispheric imbalance in stroke patients as revealed by combining transcranial magnetic stimulation and electroencephalography. Human Brain Mapp. 2020, 1–16. [Google Scholar] [CrossRef]





| Averaged iSP Duration (ms)/IAR | Anatomical Imitation | Specular Imitation | No Imitation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LH–RH | RH–LH | IAR | LH–RH | RH–LH | IAR | LH–RH | RH–LH | IAR | |
| Pre (baseline) | 28.86 | 27.52 | 1.07 | 26.97 | 27.03 | 1.01 | 27.69 | 25.79 | 1.09 |
| (6.46) | (4.50) | (0.25) | (4.33) | (3.51) | (0.17) | (6.80) | (4.02) | (0.27) | |
| NDH imitation | 29.59 | 28.45 | 1.07 | 29.07 | 28.48 | 1.05 | 27.83 | 31.79 | 0.91 |
| (6.77) | (7.02) | (0.27) | (5.11) | (6.40) | (0.19) | (6.98) | (8.52) | (0.25) | |
| DH imitation | 28.21 | 28.00 | 1.03 | 29.21 | 28.07 | 1.06 | 29.79 | 30.10 | 1.03 |
| (5.92) | (5.11) | (0.27) | (4.85) | (4.72) | (0.22) | (7.97) | (8.92) | (0.27) | |
| Δ NDH imitation | 0.72 | 0.93 | 0.005 | 2.10 | 1.45 | 0.04 | 0.14 | 6.00 | –0.18 |
| (5.13) | (7.26) | (0.30) | (6.12) | (6.39) | (0.18) | (6.20) | (9.41) | (0.39) | |
| Δ DH imitation | –0.65 | 0.48 | –0.03 | 2.24 | 1.03 | 0.05 | 2.10 | 4.31 | –0.06 |
| (5.14) | (6.11) | (0.30) | (5.62) | (6.39) | (0.26) | (6.84) | (8.88) | (0.39) | |
| Post | 24.76 | 24.31 | 1.02 | 26.38 | 26.24 | 1.02 | 28.52 | 26.69 | 1.09 |
| (5.99) | (4.17) | (0.19) | (5.22) | (4.40) | (0.17) | (7.51) | (4.91) | (0.26) | |
| Later | 26.41 | 26.62 | 1.00 | 27.31 | 26.24 | 1.06 | 28.83 | 27.31 | 1.09 |
| (4.97) | (4.16) | (0.17) | (4.36) | (4.48) | (0.22) | (6.35) | (6.47) | (0.26) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, D.; Izumi, S.-i.; Suzuki, E. Modulation of Interhemispheric Inhibition between Primary Motor Cortices Induced by Manual Motor Imitation: A Transcranial Magnetic Stimulation Study. Brain Sci. 2021, 11, 266. https://doi.org/10.3390/brainsci11020266
Tian D, Izumi S-i, Suzuki E. Modulation of Interhemispheric Inhibition between Primary Motor Cortices Induced by Manual Motor Imitation: A Transcranial Magnetic Stimulation Study. Brain Sciences. 2021; 11(2):266. https://doi.org/10.3390/brainsci11020266
Chicago/Turabian StyleTian, Dongting, Shin-ichi Izumi, and Eizaburo Suzuki. 2021. "Modulation of Interhemispheric Inhibition between Primary Motor Cortices Induced by Manual Motor Imitation: A Transcranial Magnetic Stimulation Study" Brain Sciences 11, no. 2: 266. https://doi.org/10.3390/brainsci11020266
APA StyleTian, D., Izumi, S.-i., & Suzuki, E. (2021). Modulation of Interhemispheric Inhibition between Primary Motor Cortices Induced by Manual Motor Imitation: A Transcranial Magnetic Stimulation Study. Brain Sciences, 11(2), 266. https://doi.org/10.3390/brainsci11020266