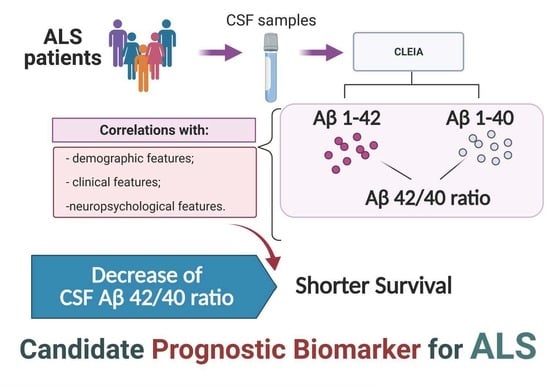
Prognostic Role of CSF β-amyloid 1–42/1–40 Ratio in Patients Affected by Amyotrophic Lateral Sclerosis
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. CSF Collection and Analytical Techniques
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Es, M.A.; Hardiman, O. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Brooks, B.R.; Miller, R.G. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral. Scler. Other Motor. Neuron. Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Julien, J.P. A role for neurofilaments in the pathogenesis of amyotrophic lateral sclerosis. Biochem. Cell Biol. 1995, 73, 593–597. [Google Scholar] [CrossRef]
- Didonna, A.; Opal, P. The role of neurofilament aggregation in neurodegeneration: Lessons from rare inherited neurological disorders. Mol. Neurodegener. 2019, 14, 19. [Google Scholar] [CrossRef]
- Steinacker, P.; Feneberg, E. Neurofilaments in the diagnosis of motoneuron diseases: A prospective study on 455 patients. J. Neurol. Neurosurg. Psychiatry 2016, 87, 12–20. [Google Scholar] [CrossRef]
- Rossi, D.; Volanti, P. CSF neurofilament proteins as diagnostic and prognostic biomarkers for amyotrophic lateral sclerosis. J. Neurol. 2018, 265, 510–521. [Google Scholar] [CrossRef]
- Paladino, P.; Valentino, F. Cerebrospinal fluid tau protein is not a biological marker in amyotrophic lateral sclerosis. Eur. J. Neur. 2009, 16, 257–261. [Google Scholar] [CrossRef]
- Grossman, M.; Elman, L.; McCluskey, L. Phosphorylated tau as a candidate biomarker for amyotrophic lateral sclerosis. JAMA Neurol. 2014, 71, 442–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilke, C.; Deuschle, C. Total tau is increased, but phosphorylated tau not decreased, in cerebrospinal fluid in amyotrophic lateral sclerosis. Neurobiol. Aging 2015, 36, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Burrell, J.R.; Halliday, G.M. The frontotemporal dementia-motor neuron disease continuum. Lancet 2016, 388, 919–931. [Google Scholar] [CrossRef]
- DeJesus-Hernandez, M.; Mackenzie, I.R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 2011, 72, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Renton, A.E.; Majounie, E. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011, 72, 257–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calingasan, N.Y.; Chen, J. Β-Amyloid 42 Accumulation in the Lumbar Spinal Cord Motor Neurons of Amyotrophic Lateral Sclerosis Patients. Neurobiol. Dis. 2005, 19, 340–347. [Google Scholar] [CrossRef]
- Farid, K.; Carter, S.F.; Rodriguez-Vieitez, E. Case Report of Complex Amyotrophic Lateral Sclerosis with Cognitive Impairment and Cortical Amyloid Deposition. JAD 2015, 47, 661–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, S.; Iwata, M. Immunoreactivity of beta-amyloid precursor protein in amyotrophic lateral sclerosis. Acta Neuropathol. 1999, 97, 463–468. [Google Scholar] [CrossRef]
- Gómez-Pinedo, U.; Villar-Quiles, R.N. Immununochemical Markers of the Amyloid Cascade in the Hippocampus in Motor Neuron Diseases. Front Neurol. 2016, 7, 195. [Google Scholar] [CrossRef] [Green Version]
- Hardy, J. Alzheimer’s disease: The amyloid cascade hypothesis—An update and reappraisal. JAD 2009, 9, 151–153. [Google Scholar] [CrossRef] [Green Version]
- Jack, C.R.; Bennett, D.A. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recom- mendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [Green Version]
- McKhann, G.M.; Knopman, D.S. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association work- groups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eun, J.Y.; Hyo-Jin, P. Intracellular amyloid beta interacts with SOD1 and impairs the enzymatic activity of SOD1: Implications for the pathogenesis of amyotrophic lateral sclerosis. Exp. Mol. Med. 2009, 41, 611–617. [Google Scholar]
- Stanga, S.; Brambilla, L. A role for GDNF and soluble APP as biomarkers of amyotrophic lateral sclerosis pathophysiology. Front. Neurol. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Steinacker, P.; Hendrich, C. Concentrations of beta-amyloid precursor protein processing products in cerebrospinal fluid of patients with amyotrophic lateral sclerosis and frontotemporal lobar degeneration. J. Neural. Transm. 2009, 116, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Rusina, R.; Ridzon, P. Relationship between ALS and the degree of cognitive impairment, markers of neurodegeneration and predictors for poor outcome. A prospective study. Eur. J. Neurol. 2010, 17, 23–30. [Google Scholar] [CrossRef]
- Lanznaster, D.; Hergesheimer, R.C. Aβ1-42 and Tau as Potential Biomarkers for Diagnosis and Prognosis of Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020, 21, 2911. [Google Scholar] [CrossRef] [Green Version]
- Hadjichrysanthou, C.; Evans, S. The dynamics of biomarkers across the clinical spectrum of Alzheimer’s disease. Alzheimers Res. Ther. 2020, 12, 74. [Google Scholar] [CrossRef]
- Hall, S.; Surova, Y. CSF biomarkers and clinical progression of Parkinson disease. Neurology 2015, 84, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Hansson, O.; Seibyl, J. CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement 1470, 14, 1470–1481. [Google Scholar] [CrossRef]
- Li, Q.X.; Mok, S.S. Overexpression of Abeta is associated with acceleration of onset of motor impairment and superoxide dismutase 1 aggregation in an amyotrophic lateral sclerosis mouse model. Aging Cell. 2006, 5, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, M.; Davidsson, P. Decreased CSF-beta-amyloid 42 in Alzheimer’s disease and amyotrophic lateral sclerosis may reflect mismetabolism of beta-amyloid induced by disparate mechanisms. Dement. Geriatr. Cogn. Disord. 2002, 13, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich-Toidman, P.; Becker, M. Inhibition of amyloid precursor protein beta-secretase cleavage site affects survival and motor functions of amyotrophic lateral sclerosis transgenic mice. Neurodegener. Dis. 2012, 10, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Chiò, A.; Logroscino, G. Prognostic factors in ALS: A critical review. Amyotroph. Lateral. Scler. 2009, 10, 310–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paternicò, D.; Galluzzi, S. Cerebrospinal fluid markers for Alzheimer’s disease in a cognitively healthy cohort of young and old adults. Alzheimer’s Dement. 2012, 8, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Rodrigue, K.M.; Kennedy, K.M. Beta-amyloid deposition and the aging brain. Neuropsychol. Rev. 2009, 19, 436–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Variables | ALS tot (n = 93) | ALS-s (n = 19) | ALS-i (n = 31) | ALS-r (n = 35) | p |
|---|---|---|---|---|---|
| Age at onset (years) | 67 (63–72) | 63 (61–67) | 67 (64–72) | 70 (64–74) | < 0.001 * |
| M/F | 1.11 | 2.16 | 1 | 1.18 | 0.43 ** χ2 = 1.67 with 2 DF |
| Education (years) | 5 (5–13) | 13 (5–13) | 8 (5–8) | 5 (5–9) | 0.283 * |
| Type of onset familiar, % sporadic, % | 3.2% 96.6% | 5.2% 94.8% | 3.3% 96.7% | 2.8% 97.2% | 0.90 ** χ2 = 0.22 with 2 DF |
| Site of onset Spinal, % Bulbar, % | 70.3% 29.7% | 89.5% 10.5% | 63.3% 36.7% | 62.3% 37.2% | 0.09 ** χ2 = 4.80 with 2 DF |
| Diagnostic delay (months) | 12 (9–20) | 25 (18–37) | 12 (10–24) | 7 (4–9.5) | < 0.001 * |
| Rate of progression (ΔFS) A | 0.8 (0.5–1.3) | – | – | – | – |
| FVC a (%) | 81 (55–93) | 84 (59–98) | 83 (60–92) | 67 (46–93) | 0.334 * |
| BMI b (kg/m2) | 24.8 (21.5–27.1) | 25 (21–28) | 24 (22–27) | 25.7 (46–83) | 0.355 * |
| Survival (months) | 30 (20–46) | 57 (37–67) | 35 (27–47) | 17 (13–26) | < 0.001 * |
| Parameters | ALS tot (n = 93) | ALS-s (n = 19) | ALS-i (n = 31) | ALS-r (n = 35) | p * |
|---|---|---|---|---|---|
| Proteins (mg/dL) | 39 (28–51) | 37 (19–52) | 39 (32–62) | 37 (26–48) | 0.524 |
| Glucose (mg/dL) | 60 (55–66) | 58 (55–63) | 56 (51–66) | 62 (57–72) | 0.103 |
| Cells (lymphocytes) | 0.8 (0.6–1.8) | 0.8 (0.6–2.3) | 1 (0.6–2.9) | 0.8 (0.4–1.6) | 0.371 |
| Oligoclonal bands (y/n)** | 17/76 | 4/15 | 4/27 | 7/28 |
| Aβ 1–42 | Aβ 1–40 | |
|---|---|---|
| Age at onset (years) | r = −0.041, p = 0.695 | r = 0.312, p = 0.208 |
| Diagnostic delay (months) | r = −0.140, p = 0.189 | r = –0.163, p = 0.126 |
| Rate of progression (ΔFS) | r = 0.008 p = 0.936 | r = 0.103, p = 0.347 |
| FVC (%) | r = 0.141, p = 0.237 | r = 0.116, p = 0.330 |
| FrSBe | r = 0.125, p = 0.370 | r = 0.185, p = 0.196 |
| MMSE | r = −0.240, p = 0.146 | r = −0.429, p = 0.007 |
| ECAS | r = 0.005, p = 0.979 | r = −0.049, p = 0.304 |
| Survival (months) | r = 0.106, p = 0.933 | r = −0.119, p = 0.350 |
| Parameters | r2 | p |
|---|---|---|
| Age at onset (years) | −0.274 | 0.008 |
| Diagnostic delay (months) | 0.038 | 0.719 |
| ΔFS | −0.086 | 0.432 |
| FVC(%) | 0.198 | 0.095 |
| FrSBe | −0.076 | 0.695 |
| MMSE | 0.396 | 0.019 |
| ECAS | 0.054 | 0.792 |
| Survival (months) | 0.164 | 0.196 |
| Parameters | b | ± SE | p | HR | 95% CI |
|---|---|---|---|---|---|
| Gender (M vs. F) | 0.073 | 0.251 | 0.772 | 1.075 | 0.658–1.757 |
| Age at onset | 0.062 | 0.019 | 0.001 | 1.064 | 1.024–1.105 |
| Site of onset (spinal vs. bulbar) | −0.443 | 0.271 | 0.102 | 0.642 | 0.378–1.092 |
| Diagnostic delay | −0.038 | 0.012 | 0.001 | 0.963 | 0.000–0.986 |
| FVC% | 0.006 | 0.006 | 0.308 | 0.994 | 0.982–1.006 |
| ΔFS at diagnosis | 0.443 | 0.105 | < 0.001 | 1.557 | 1.266–1.914 |
| Aβ 1–42 | 0.000 | 0.000 | 0.862 | 1 | 0.999–1.001 |
| Aβ 1–40 | 0.000 | 0.000 | 0.275 | 1 | 1–1 |
| Aβ 42/40 ratio | −18.137 | 8.164 | 0.026 | 1.33 × 10–8 | 0.000–0.118 |
| Parameters | b | ± SE | p | HR | 95% CI |
|---|---|---|---|---|---|
| Age at onset | 0.038 | 0.022 | 0.08 | 1.038 | 0.994–1.085 |
| Diagnostic delay | −0.032 | 0.014 | 0.025 | 0.968 | 0.000–0.996 |
| ΔFS at diagnosis | 0.301 | 0.140 | 0.032 | 1.351 | 1.026–1.779 |
| Aβ 42/40 ratio | −20.662 | 8.504 | 0.015 | 1.6 × 10–9 | 0.000–0.018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colletti, T.; Agnello, L.; Spataro, R.; Guccione, L.; Notaro, A.; Lo Sasso, B.; Blandino, V.; Graziano, F.; Gambino, C.M.; Giglio, R.V.; et al. Prognostic Role of CSF β-amyloid 1–42/1–40 Ratio in Patients Affected by Amyotrophic Lateral Sclerosis. Brain Sci. 2021, 11, 302. https://doi.org/10.3390/brainsci11030302
Colletti T, Agnello L, Spataro R, Guccione L, Notaro A, Lo Sasso B, Blandino V, Graziano F, Gambino CM, Giglio RV, et al. Prognostic Role of CSF β-amyloid 1–42/1–40 Ratio in Patients Affected by Amyotrophic Lateral Sclerosis. Brain Sciences. 2021; 11(3):302. https://doi.org/10.3390/brainsci11030302
Chicago/Turabian StyleColletti, Tiziana, Luisa Agnello, Rossella Spataro, Lavinia Guccione, Antonietta Notaro, Bruna Lo Sasso, Valeria Blandino, Fabiola Graziano, Caterina Maria Gambino, Rosaria Vincenza Giglio, and et al. 2021. "Prognostic Role of CSF β-amyloid 1–42/1–40 Ratio in Patients Affected by Amyotrophic Lateral Sclerosis" Brain Sciences 11, no. 3: 302. https://doi.org/10.3390/brainsci11030302
APA StyleColletti, T., Agnello, L., Spataro, R., Guccione, L., Notaro, A., Lo Sasso, B., Blandino, V., Graziano, F., Gambino, C. M., Giglio, R. V., Bivona, G., La Bella, V., Ciaccio, M., & Piccoli, T. (2021). Prognostic Role of CSF β-amyloid 1–42/1–40 Ratio in Patients Affected by Amyotrophic Lateral Sclerosis. Brain Sciences, 11(3), 302. https://doi.org/10.3390/brainsci11030302