Visual Event-Related Potentials under External Emotional Stimuli in Bipolar I Disorder with and without Hypersexuality
Abstract
:1. Introduction
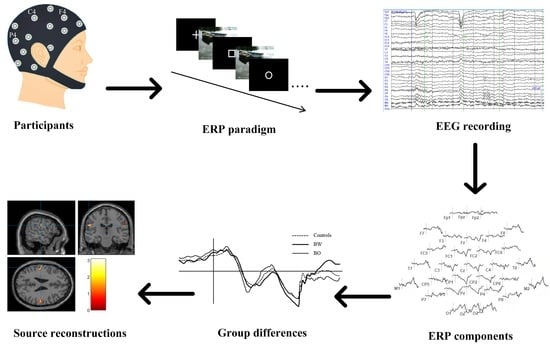
2. Materials and Methods
2.1. Participants
2.2. Questionnaires
2.3. ERP Designs and Recordings
2.3.1. External Emotional Stimuli
2.3.2. ERP Paradigm
2.3.3. ERP Recording
2.4. Statistical Analyses
3. Results
3.1. Concurrent Affective States and Behavioral Results
3.2. ERP Components
3.3. Source Reconstructions
3.4. Relationships between ERPs and Concurrent Affective States
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elliott, R.; Zahn, R.; Deakin, J.F.; Anderson, I.M. Affective cognition and its disruption in mood disorders. Neuropsychopharmacology 2011, 36, 153–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solé, B.; Jiménez, E.; Torrent, C.; Reinares, M.; Bonnin, C.; Torres, I.; Varo, C.; Grande, I.; Valls, E.; Salagre, E.; et al. Cognitive Impairment in Bipolar Disorder: Treatment and Prevention Strategies. Int. J. Neuropsychopharmacol. 2017, 20, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Vieta, E.; Berk, M.; Schulze, T.G.; Carvalho, A.F.; Suppes, T.; Calabrese, J.R.; Gao, K.; Miskowiak, K.W.; Grande, I. Bipolar disorders. Nat. Rev. Dis. Primers 2018, 4, 18008. [Google Scholar] [CrossRef] [PubMed]
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar disorder. Lancet 2016, 387, 1561–1572. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Berk, M.; Brietzke, E.; Goldstein, B.I.; López-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A.; et al. Bipolar disorders. Lancet 2020, 396, 1841–1856. [Google Scholar] [CrossRef]
- Małgorzata, P.; Paweł, K.; Iwona, M.L.; Brzostek, T.; Andrzej, P. Glutamatergic dysregulation in mood disorders: Opportunities for the discovery of novel drug targets. Expert Opin. Ther. Targets 2020, 24, 1187–1209. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Serretti, A.; Olgiati, P. Profiles of “manic” symptoms in bipolar I, bipolar II and major depressive disorders. J. Affect. Disord. 2005, 84, 159–166. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Press: Arlington, VA, USA, 2013. [Google Scholar]
- Sagar, K.A.; Dahlgren, M.K.; Gönenç, A.; Gruber, S.A. Altered affective processing in bipolar disorder: An fMRI study. J. Affect. Disord. 2013, 150, 1192–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepede, G.; De Berardis, D.; Campanella, D.; Perrucci, M.G.; Ferretti, A.; Salerno, R.M.; Di Giannantonio, M.; Romani, G.L.; Gambi, F. Neural correlates of negative emotion processing in bipolar disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 60, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gruber, J.; Kogan, A.; Mennin, D.; Murray, G. Real-world emotion? an experience-sampling approach to emotion experience and regulation in bipolar I disorder. J. Abnorm. Psychol. 2013, 122, 971–983. [Google Scholar] [CrossRef] [Green Version]
- Hoertnagl, C.M.; Muehlbacher, M.; Biedermann, F.; Yalcin, N.; Baumgartner, S.; Schwitzer, G.; Deisenhammer, E.A.; Hausmann, A.; Kemmler, G.; Benecke, C.; et al. Facial emotion recognition and its relationship to subjective and functional outcomes in remitted patients with bipolar I disorder. Bipolar Disord. 2011, 13, 537–544. [Google Scholar] [CrossRef]
- Lima, I.M.M.; Peckham, A.D.; Johnson, S.L. Cognitive deficits in bipolar disorders: Implications for emotion. Clin. Psychol. Rev. 2018, 59, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, C.; Sundet, K.; Vaskinn, A.; Birkenaes, A.B.; Engh, J.A.; Hansen, C.F.; Jonsdottir, H.; Ringen, P.A.; Opjordsmoen, S.; Friis, S.; et al. Neurocognitive profiles in bipolar I and bipolar II disorder: Differences in pattern and magnitude of dysfunction. Bipolar Disord. 2008, 10, 245–255. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Serio, G.; Scarpazza, C.; D’Ausilio, A.; Borgomaneri, S. Frozen in (e)motion: How reactive motor inhibition is influenced by the emotional content of stimuli in healthy and psychiatric populations. Behav. Res. Ther. 2021, 146, 103963. [Google Scholar] [CrossRef]
- Hıdıroğlu, C.; Torres, I.J.; Er, A.; Işık, G.; Yalın, N.; Yatham, L.N.; Ceylan, D.; Özerdem, A. Response inhibition and interference control in patients with bipolar I disorder and first-degree relatives. Bipolar Disord. 2015, 17, 781–794. [Google Scholar] [CrossRef]
- Tsujii, N.; Mikawa, W.; Adachi, T.; Hirose, T.; Shirakawa, O. Shared and differential cortical functional abnormalities associated with inhibitory control in patients with schizophrenia and bipolar disorder. Sci. Rep. 2018, 8, 4686. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Serio, G.; Battaglia, S. Please, don’t do it! Fifteen years of progress of non-invasive brain stimulation in action inhibition. Cortex 2020, 132, 404–422. [Google Scholar] [CrossRef]
- Sharp, D.J.; Bonnelle, V.; De Boissezon, X.; Beckmann, C.F.; James, S.G.; Patel, M.C.; Mehta, M.A. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc. Natl. Acad. Sci. USA 2010, 107, 6106–6111. [Google Scholar] [CrossRef] [Green Version]
- Battaglia, S. Neurobiological advances of learned fear in humans. Adv. Clin. Exp. Med. 2022. [Google Scholar] [CrossRef]
- Dixon, M.L.; Thiruchselvam, R.; Todd, R.; Christoff, K. Emotion and the prefrontal cortex: An integrative review. Psychol. Bull. 2017, 143, 1033–1081. [Google Scholar] [CrossRef] [PubMed]
- Kopeykina, I.; Kim, H.J.; Khatun, T.; Boland, J.; Haeri, S.; Cohen, L.J.; Galynker, I.I. Hypersexuality and couple relationships in bipolar disorder: A review. J. Affect. Disord. 2016, 195, 1–14. [Google Scholar] [CrossRef]
- Bőthe, B.; Tóth-Király, I.; Potenza, M.N.; Griffiths, M.D.; Orosz, G.; Demetrovics, Z. Revisiting the role of impulsivity and compulsivity in problematic sexual behaviors. J. Sex Res. 2019, 56, 166–179. [Google Scholar] [CrossRef] [Green Version]
- Reid, R.C.; Bramen, J.E.; Anderson, A.; Cohen, M.S. Mindfulness, emotional dysregulation, impulsivity, and stress proneness among hypersexual patients. J. Clin. Psychol. 2014, 70, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jardin, C.; Sharp, C.; Garey, L.; Vanwoerden, S.; Crist, N.; Elhai, J.D.; Zvolensky, M.J. Compelled to risk: Does sexual compulsivity explain the connection between borderline personality disorder features and number of sexual partners? J. Pers. Disord. 2017, 31, 738–752. [Google Scholar] [CrossRef]
- Lloyd, M.; Raymond, N.C.; Miner, M.H.; Coleman, E. Borderline personality traits in individuals with compulsive sexual behavior. Sex Addict. Compulsivity 2007, 14, 187–206. [Google Scholar] [CrossRef]
- Di Nicola, M.; Tedeschi, D.; Mazza, M.; Martinotti, G.; Harnic, D.; Catalano, V.; Bruschi, A.; Pozzi, G.; Bria, P.; Janiri, L. Behavioural addictions in bipolar disorder patients: Role of impulsivity and personality dimensions. J. Affect. Disord. 2010, 125, 82–88. [Google Scholar] [CrossRef]
- Lew-Starowicz, M.; Lewczuk, K.; Nowakowska, I.; Kraus, S.; Gola, M. Compulsive sexual behavior and dysregulation of emotion. Sex Med. Rev. 2020, 8, 191–205. [Google Scholar] [CrossRef]
- Kafka, M.P. Hypersexual disorder: A proposed diagnosis for DSM-V. Arch. Sex Behav. 2010, 39, 377–400. [Google Scholar] [CrossRef]
- Werner, M.; Štulhofer, A.; Waldorp, L.; Jurin, T. A network approach to hypersexuality: Insights and clinical implications. J. Sex Med. 2018, 15, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Gopin, C.B.; Burdick, K.E.; DeRosse, P.; Goldberg, T.E.; Malhotra, A.K. Emotional modulation of response inhibition in stable patients with bipolar I disorder: A comparison with healthy and schizophrenia subjects. Bipolar Disord. 2011, 13, 164–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soncin, S.; Brien, D.C.; Coe, B.C.; Marin, A.; Munoz, D.P. Contrasting emotion processing and executive functioning in Attention-Deficit/Hyperactivity disorder and bipolar disorder. Behav. Neurosci. 2016, 130, 531–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, A.K. Affective influences on the attentional dynamics supporting awareness. J. Exp. Psychol. Gen. 2005, 134, 258–281. [Google Scholar] [CrossRef] [Green Version]
- Gross, J.J. The emerging field of emotion regulation: An integrative review. Rev. Gen. Psychol. 1998, 2, 271–299. [Google Scholar] [CrossRef]
- Rocca, C.C.; Heuvel, E.v.; Caetano, S.C.; Lafer, B. Facial emotion recognition in bipolar disorder: A critical review. Braz. J. Psychiatry 2009, 31, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Cusi, A.M.; Nazarov, A.; Holshausen, K.; Macqueen, G.M.; McKinnon, M.C. Systematic review of the neural basis of social cognition in patients with mood disorders. J. Psychiatry Neurosci. 2012, 37, 154–169. [Google Scholar] [CrossRef] [Green Version]
- García-Blanco, A.; Salmerón, L.; Perea, M. Inhibitory control for emotional and neutral scenes in competition: An eye-tracking study in bipolar disorder. Biol. Psychol. 2017, 127, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Ryu, V.; An, S.K.; Jo, H.H.; Cho, H.S. Decreased P3 amplitudes elicited by negative facial emotion in manic patients: Selective deficits in emotional processing. Neurosci. Lett. 2010, 481, 92–96. [Google Scholar] [CrossRef]
- Wang, C.; Shao, X.; Jia, Y.; Ho, R.C.; Harris, K.M.; Wang, W. Peripherally physiological responses to external emotions and their transitions in bipolar I disorder with and without hypersexuality. Arch. Sex Behav. 2020, 49, 1345–1354. [Google Scholar] [CrossRef]
- Helfrich, R.F.; Knight, R.T. Cognitive neurophysiology: Event-related potentials. In Handbook of Clinical Neurology; Levin, K.H., Chauvel, P., Eds.; Elsevier: Cambridge, UK, 2019; pp. 543–558. [Google Scholar]
- Lijffijt, M.; Lane, S.D.; Meier, S.L.; Boutros, N.N.; Burroughs, S.; Steinberg, J.L.; Gerard Moeller, F.; Swann, A.C. P50, N100, and P200 sensory gating: Relationships with behavioral inhibition, attention, and working memory. Psychophysiology 2009, 46, 1059–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wascher, E.; Hoffmann, S.; Sänger, J.; Grosjean, M. Visuo-spatial processing and the N1 component of the ERP. Psychophysiology 2009, 46, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.H.; Azzam, P.N. Characterization of N200 and P300: Selected studies of the event-related potential. Int. J. Med. Sci. 2005, 2, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [Green Version]
- Hirschfeld, R.M.A.; Williams, J.B.W.; Spitzer, R.L.; Calabrese, J.R.; Flynn, L.; Keck, P.E.J.; Lewis, L.; McElroy, S.L.; Post, R.M.; Rapport, D.J.; et al. Development and validation of a screening instrument for bipolar spectrum disorder: The mood disorder questionnaire. Am. J. Psychiatry 2000, 157, 1873–1875. [Google Scholar] [CrossRef]
- Angst, J.; Adolfsson, R.; Benazzi, F.; Gamma, A.; Hantouche, E.; Meyer, T.D.; Skeppar, P.; Vieta, E.; Scott, J. The HCL-32: Towards a self-assessment tool for hypomanic symptoms in outpatients. J. Affect. Disord. 2005, 88, 217–233. [Google Scholar] [CrossRef]
- Plutchik, R.; van Praag, H.M. Interconvertability of five self-report measures of depression. Psychiatry Res. 1987, 22, 243–256. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual; Technical Report A-8; University of Florida: Gainesville, FL, USA, 2008. [Google Scholar]
- Bradley, M.M.; Lang, P.J. The International Affective Digitized Sounds (IADS-2): Affective Ratings of Sounds and Instruction Manual; Technical Report B-3; University of Florida: Gainesville, FL, USA, 2007. [Google Scholar]
- Zhang, B.; Jia, Y.; Wang, C.; Shao, X.; Wang, W. Visual event-related potentials in external emotional conditions in bipolar disorders I and II. Neurophysiol. Clin. 2019, 49, 359–369. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.; Huang, W.; Hu, S. Brain Effective Connectivity Analysis from EEG for Positive and Negative Emotion. In Neural Information Processing; Liu, D., Xie, S., Li, Y., Zhao, D., El-Alfy, E.S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 851–857. [Google Scholar]
- Dolcos, F.; LaBar, K.S.; Cabeza, R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: An event-related fMRI study. Neuroimage 2004, 23, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.W.; Vartanian, O.; Goel, V. Dissociable neural systems underwrite logical reasoning in the context of induced emotions with positive and negative valence. Front. Hum. Neurosci. 2014, 8, 736. [Google Scholar] [CrossRef] [Green Version]
- Lennox, B.R.; Jacob, R.; Calder, A.J.; Lupson, V.; Bullmore, E.T. Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychol. Med. 2004, 34, 795–802. [Google Scholar] [CrossRef]
- Reid, R.C. Differentiating emotions in a sample of men in treatment for hypersexual behavior. J. Soc. Work Pract. Addict. 2010, 10, 197–213. [Google Scholar] [CrossRef]
- Kropf, E.; Syan, S.K.; Minuzzi, L.; Frey, B.N. From anatomy to function: The role of the somatosensory cortex in emotional regulation. Braz. J. Psychiatry 2019, 41, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Corbetta, M.; Patel, G.; Shulman, G.L. The reorienting system of the human brain: From environment to theory of mind. Neuron 2008, 58, 306–324. [Google Scholar] [CrossRef] [Green Version]
- Silani, G.; Lamm, C.; Ruff, C.C.; Singer, T. Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. J. Neurosci. 2013, 33, 15466–15476. [Google Scholar] [CrossRef]
- Hampshire, A.; Chamberlain, S.R.; Monti, M.M.; Duncan, J.; Owen, A.M. The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage 2010, 50, 1313–1319. [Google Scholar] [CrossRef] [Green Version]
- Tabei, K. Inferior frontal gyrus activation underlies the perception of emotions, while precuneus activation underlies the feeling of emotions during music listening. Behav. Neurol. 2015, 2015, 529043. [Google Scholar] [CrossRef] [Green Version]
- Paunovic, N.; Hallberg, J. Conceptualization of hypersexual disorder with the behavioral-cognitive inhibition theory. Psychology 2014, 5, 151–159. [Google Scholar] [CrossRef] [Green Version]
- García-Blanco, A.; Salmerón, L.; Perea, M. Attentional capture by emotional scenes across episodes in bipolar disorder: Evidence from a free-viewing task. Biol. Psychol. 2015, 108, 36–42. [Google Scholar] [CrossRef]
- Lembke, A.; Ketter, T.A. Impaired recognition of facial emotion in mania. Am. J. Psychiatry 2002, 159, 302–304. [Google Scholar] [CrossRef]
- Kozlovskiy, S.A.; Pyasik, M.M.; Korotkova, A.V.; Vartanov, A.V.; Glozman, J.M.; Kiselnikov, A.A. Activation of left lingual gyrus related to working memory for schematic faces. Int. J. Psychophysiol. 2014, 94, 241. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Gross, J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005, 9, 242–249. [Google Scholar] [CrossRef]
- Talati, A.; Hirsch, J. Functional specialization within the medial frontal gyrus for perceptual go/no-go decisions based on “what,” “when,” and “where” related information: An fMRI study. J. Cogn. Neurosci. 2014, 17, 981–993. [Google Scholar] [CrossRef]
- Mruczek, R.E.B.; Sheinberg, D.L. Activity of inferior temporal cortical neurons predicts recognition choice behavior and recognition time during visual search. J. Neurosci. 2007, 27, 2825–2836. [Google Scholar] [CrossRef]
- Begemann, M.J.; Brand, B.A.; Ćurčić-Blake, B.; Aleman, A.; Sommer, I.E. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: A meta-analysis. Psychol. Med. 2020, 50, 2465–2486. [Google Scholar] [CrossRef]
- Schulz, R.; Gerloff, C.; Hummel, F.C. Non-invasive brain stimulation in neurological diseases. Neuropharmacology 2013, 64, 579–587. [Google Scholar] [CrossRef]
- Yamada, Y.; Inagawa, T.; Hirabayashi, N.; Sumiyoshi, T. Emotion Recognition Deficits in Psychiatric Disorders as a Target of Non-invasive Neuromodulation: A Systematic Review. Clin. EEG Neurosci. 2021, 1550059421991688. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Garofalo, S.; Tortora, F.; Avenanti, A.; di Pellegrino, G. State-Dependent TMS over Prefrontal Cortex Disrupts Fear-Memory Reconsolidation and Prevents the Return of Fear. Curr. Biol. 2020, 30, 3672–3679.e4. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Sciamanna, G.; Tortora, F.; Laricchiuta, D. Memories are not written in stone: Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Neurosci. Biobehav. Rev. 2021, 127, 334–352. [Google Scholar] [CrossRef]






| Controls | BW | BO | |
|---|---|---|---|
| MDQ | 5.61 ± 3.19 | 9.74 ± 1.70 a | 9.12 ± 1.45 a |
| HCL-32 | 15.91 ± 3.42 | 22.48 ± 2.47 a | 21.77 ± 1.53 a |
| PVP | 13.30 ± 6.62 | 11.52 ± 7.12 | 12.08 ± 6.26 |
| Reaction time (ms) | |||
| under Disgust | 489.45 ± 85.85 | 501.94 ± 57.37 | 517.96 ± 104.08 |
| under Erotica | 516.68 ± 99.59 | 541.12 ± 69.88 | 536.45 ± 117.45 |
| under Fear | 496.19 ± 83.93 | 536.20 ± 88.27 | 531.39 ± 105.95 |
| under Happiness | 489.81 ± 82.42 | 516.56 ± 60.61 | 513.74 ± 100.08 |
| under Neutral | 489.06 ± 84.97 | 532.34 ± 83.78 | 512.94 ± 112.24 |
| under Sadness | 498.96 ± 88.48 | 513.14 ± 66.33 | 539.83 ± 111.03 |
| Response accuracy (%) | |||
| under Disgust | 94.18 ± 7.00 | 97.04 ± 2.97 | 93.61 ± 7.67 |
| under Erotica | 97.23 ± 5.62 | 94.32± 12.67 | 97.22 ± 5.70 |
| under Fear | 97.59 ± 5.93 | 97.04 ± 6.36 | 96.53 ± 9.55 |
| under Happiness | 95.46 ± 13.05 | 96.42 ± 8.16 | 99.17 ± 2.25 |
| under Neutral | 98.30 ± 3.60 | 96.05 ± 7.90 | 98.75 ± 2.37 |
| under Sadness | 98.08 ± 4.95 | 95.06 ± 11.82 | 97.36 ± 3.68 |
| Controls | BW | BO | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| Control-BW | Control-BO | BW-BO | |||||
| N1 latency (ms) | |||||||
| Erotica | F3 | 115.59 ± 21.74 | 136.36 ± 30.50 a | 115.50 ± 21.89 b | −34.69~−6.86 | −14.00~14.18 | 4.65~37.09 |
| Fz | 112.60 ± 20.29 | 131.84 ± 30.09 a | 113.54 ± 24.65 b | −33.08~−5.40 | −14.95~13.08 | 2.17~34.43 | |
| F4 | 117.02 ± 22.49 | 135.10 ± 30.48 a | 118.09 ± 22.52 b | −32.27~−3.89 | −15.44~13.30 | 0.47~33.55 | |
| C3 | 116.00 ± 23.88 | 138.71 ± 30.39 a | 113.81 ± 24.16 b | −37.46~−7.96 | −12.74~17.14 | 7.71~42.11 | |
| Cz | 114.34 ± 22.00 | 136.76 ± 28.96 a | 113.24 ± 22.21 b | −36.17~−8.67 | −12.83~15.02 | 7.49~39.55 | |
| C4 | 123.33 ± 25.81 | 139.69 ± 29.40 a | 119.06 ± 27.50 b | −31.94~−0.79 | −11.51~20.04 | 2.47~38.78 | |
| P3 | 130.15 ± 30.46 | 142.80 ± 28.71 | 123.08 ± 26.04 b | −29.30~3.99 | −9.79~23.92 | 0.32~39.12 | |
| Pz | 120.43 ± 27.84 | 139.87 ± 27.90 a | 124.81 ± 23.13 | −34.81~−4.07 | −19.94~11.19 | −2.86~32.98 | |
| P4 | 130.85 ± 26.04 | 144.32 ± 25.28 | 130.14 ± 25.51 | −28.22~1.28 | −14.23~15.65 | −3.02~31.37 | |
| Happiness | F3 | 122.07 ± 19.91 | 138.39 ± 26.40 a | 130.26 ± 25.47 | −29.54~−3.10 | −21.76~5.37 | −7.44~23.69 |
| Fz | 120.88 ± 19.58 | 134.63 ± 29.30 a | 127.06 ± 24.80 | −27.29~−0.21 | −20.08~7.71 | −8.38~23.51 | |
| F4 | 119.95 ± 21.91 | 142.48 ± 23.73 a | 131.20 ± 26.77 | −36.05~−8.99 | −25.13~2.64 | −4.66~27.20 | |
| C3 | 120.50 ± 23.36 | 133.54 ± 28.15 | 130.97 ± 27.00 | −27.68~1.59 | −25.49~4.55 | −14.66~19.81 | |
| Cz | 120.64 ± 21.22 | 132.53 ± 29.17 | 126.51 ± 25.52 | −25.78~2.01 | −20.13~8.39 | −10.35~22.38 | |
| C4 | 121.49 ± 23.75 | 137.56 ± 28.23 a | 134.01 ± 23.30 | −30.32~−1.81 | −27.15~2.11 | −13.25~20.33 | |
| P3 | 129.27 ± 27.59 | 140.09 ± 34.08 | 132.84 ± 25.38 | −27.40~5.75 | −20.59~13.44 | −12.27~26.76 | |
| Pz | 127.82 ± 24.53 | 135.67 ± 32.15 | 131.79 ± 25.11 | −23.22~7.51 | −19.73~11.80 | −14.20~21.98 | |
| P4 | 134.24 ± 30.23 | 145.19 ± 30.88 | 140.30 ± 23.59 | −27.58~5.69 | −23.13~11.01 | −14.70~24.47 | |
| P3b amplitude (μv) | |||||||
| Fear | F3 | 2.30 ± 2.66 | 1.90 ± 2.80 | 4.55 ± 4.53 a,b | −1.45~2.25 | −4.15~−0.35 | −4.83~−0.47 |
| Fz | 2.69 ± 3.08 | 1.47 ± 4.14 | 5.55 ± 5.24 a,b | −1.04~3.49 | −5.18~−0.54 | −6.75~−1.42 | |
| F4 | 2.68 ± 2.66 | 1.93 ± 2.55 | 5.06 ± 4.88 a,b | −1.14~2.64 | −4.32~−0.45 | −5.36~−0.91 | |
| C3 | 3.10 ± 3.39 | 2.51 ± 3.49 | 6.17 ± 5.04 a,b | −1.62~2.81 | −5.34~−0.80 | −6.27~−1.05 | |
| Cz | 3.78 ± 4.07 | 2.60 ± 3.50 | 7.14 ± 6.25 a,b | −1.43~3.79 | −6.05~−0.69 | −7.62~−1.47 | |
| C4 | 3.31 ± 3.22 | 2.50 ± 3.11 | 5.69 ±4.88 a,b | −1.28~2.91 | −4.52~−0.22 | −5.65~−0.72 | |
| P3 | 2.07 ± 3.69 | 1.72 ± 3.06 | 5.65 ± 5.62 a,b | −1.20~2.69 | −5.99~−1.17 | −6.69~−1.17 | |
| Pz | 2.55 ± 4.04 | 1.97 ± 4.59 | 6.55 ± 6.42 a,b | −2.19~3.35 | −6.84~−1.16 | −7.84~−1.32 | |
| P4 | 1.92 ± 3.26 | 1.43 ± 2.88 | 4.85 ± 4.73 a,b | −1.56~2.54 | −5.04~−0.83 | −5.84~−1.01 | |
| Sadness | F3 | 3.90 ± 4.10 | 3.04 ± 2.76 | 5.25± 3.95 | −1.30~3.02 | −3.56~0.87 | −4.75~0.34 |
| Fz | 4.21 ± 4.73 | 2.62 ± 2.29 | 5.50 ± 4.65 b | −0.84~4.01 | −3.78~1.20 | −5.73~−0.02 | |
| F4 | 3.87 ± 4.15 | 2.87 ± 2.13 | 5.71 ± 4.25 b | −1.17~3.16 | −4.06~0.38 | −5.38~−0.29 | |
| C3 | 4.60 ± 4.78 | 3.23 ± 2.63 | 6.33 ± 4.04 b | −1.02~3.75 | −4.18~0.72 | −5.91~−0.29 | |
| Cz | 5.34 ± 5.63 | 3.57 ± 2.51 | 7.28 ± 5.05 b | −1.03~4.57 | −4.81~0.94 | −7.01~−0.41 | |
| C4 | 4.31 ± 4.82 | 3.60 ± 3.16 | 5.51 ± 5.43 | −1.94~3.36 | −3.92~1.52 | −5.03~1.21 | |
| P3 | 3.33 ± 4.13 | 2.43 ± 2.94 | 5.36 ± 4.49 b | −1.38~3.16 | −4.37~0.30 | −5.60~−0.25 | |
| Pz | 3.55 ± 4.36 | 2.72 ± 2.89 | 6.56 ± 5.17 a,b | −1.61~3.27 | −5.51~−0.50 | −6.71~−0.96 | |
| P4 | 2.95 ± 4.02 | 2.52 ± 2.75 | 4.57 ± 3.92 | −1.70~2.56 | −3.81~0.57 | −4.56~0.46 |
| Component | Group | Source Region |
|---|---|---|
| Erotica under N1 (70~200 ms) | Controls | supramarginal gyri (B) (Parietal Lobe) * |
| lingual gyri (B) (Occipital Lobe) | ||
| inferior frontal gyrus (L) (Frontal Lobe) | ||
| BW | postcentral gyrus (L) (Parietal Lobe) * | |
| supramarginal gyrus (R) (Parietal Lobe) | ||
| inferior frontal gyrus (R) (Frontal Lobe) | ||
| BO | medial frontal gyri (B) (Frontal Lobe) * | |
| inferior temporal gyrus (R) (Temporal Lobe) | ||
| Happiness under N1 (70~200 ms) | Controls | inferior temporal gyrus (R) (Temporal Lobe) * |
| BW | inferior frontal gyri (B) (Frontal Lobe) * | |
| BO | superior frontal gyrus (L) (Frontal Lobe) * | |
| Fear under P3b (400~580 ms) | Controls | inferior occipital gyrus (R) (Occipital Lobe) * |
| BW | lingual gyri (B) (Occipital Lobe) * | |
| BO | medial frontal gyri (B) (Frontal Lobe) * | |
| inferior temporal gyrus (R) (Temporal Lobe) | ||
| Sadness under P3b (400~580 ms) | Controls | medial frontal gyri (B) (Frontal Lobe) * |
| inferior frontal gyrus (L) (Frontal Lobe) | ||
| inferior temporal gyrus (R) (Temporal Lobe) | ||
| superior frontal gyrus (R) (Frontal Lobe) | ||
| BW | medial frontal gyrus (L) (Frontal Lobe) * | |
| BO | inferior frontal gyrus (L) (Frontal Lobe) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Rimol, L.M.; Wang, W. Visual Event-Related Potentials under External Emotional Stimuli in Bipolar I Disorder with and without Hypersexuality. Brain Sci. 2022, 12, 441. https://doi.org/10.3390/brainsci12040441
Wang C, Rimol LM, Wang W. Visual Event-Related Potentials under External Emotional Stimuli in Bipolar I Disorder with and without Hypersexuality. Brain Sciences. 2022; 12(4):441. https://doi.org/10.3390/brainsci12040441
Chicago/Turabian StyleWang, Chu, Lars M. Rimol, and Wei Wang. 2022. "Visual Event-Related Potentials under External Emotional Stimuli in Bipolar I Disorder with and without Hypersexuality" Brain Sciences 12, no. 4: 441. https://doi.org/10.3390/brainsci12040441
APA StyleWang, C., Rimol, L. M., & Wang, W. (2022). Visual Event-Related Potentials under External Emotional Stimuli in Bipolar I Disorder with and without Hypersexuality. Brain Sciences, 12(4), 441. https://doi.org/10.3390/brainsci12040441