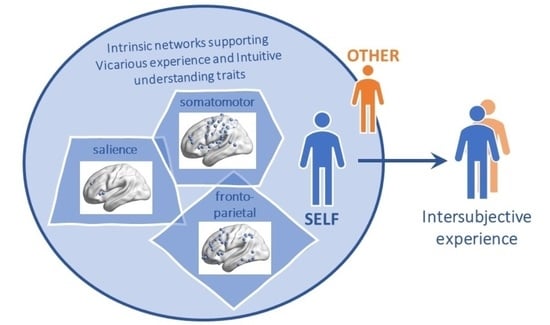
Intrinsic Shapes of Empathy: Functional Brain Network Topology Encodes Intersubjective Experience and Awareness Traits
Abstract
:1. Introduction
“To perceive is to suffer”—Aristotle
2. Materials and Methods
2.1. Participants
2.2. Empathic Experience Scale
2.3. MRI Data Acquisition and Preprocessing
2.4. MRI Data Preprocessing
2.5. fMRI Data Analysis
2.6. Analysis of Neural Networks-Empathic Traits Associations
3. Results
3.1. Behavioral Results: Empathic Experience Scale
3.2. Global Modularity
3.3. Brain Correlates of Vicarious Experience
3.4. Brain Correlates of Intuitive Understanding
4. Discussion
4.1. The Fronto-Parietal Encoding of Vicarious Experience
“I do not ask the wounded person how he feels, I myself become the wounded person.”—Walt Whitman, Song of Myself
4.2. Multiple Brain Systems Involved in Intuitive Understanding
“It’s the hardest thing in the world to go on being aware of someone else’s pain.”—Pat Barker, Life Class
4.3. Brain Networks and Empathy Concepts
4.4. Sex Effects
4.5. Limitations and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Titchner, E.B. A Textbook of Psychology; Macmillan: New York, NY, USA, 1910. [Google Scholar]
- Jahoda, G. Theodor Lipps and the shift from “sympathy” to “empathy”. J. Hist. Behav. Sci. 2005, 41, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Vischer, R. Über das Optische Formgefühl. Ein Beitrag zur Ästhetik; Hermann Credner: Leipzig, Germany, 1873. [Google Scholar]
- Husserl, E. Zur Phänomenologie der Intersubjektivität, I.; Husserliana XIII; Martinus Nijhoff: Den Haag, The Netherlands, 1973. [Google Scholar]
- Stein, E. On the Problem of Empathy; ICS Publications: Washington, DC, USA, 1989. [Google Scholar]
- Zahavi, D. Empathy, embodiment and interpersonal understanding: From Lipps to Schutz. Inquiry 2010, 53, 285–306. [Google Scholar] [CrossRef] [Green Version]
- Gallese, V. The roots of empathy: The shared manifold hypothesis and the neural basis of intersubjectivity. Psychopathology 2003, 36, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Coll, M.P.; Viding, E.; Rütgen, M.; Silani, G.; Lamm, C.; Catmur, C.; Bird, G. Are we really measuring empathy? Proposal for a new measurement framework. Neurosci. Biobehav. Rev. 2017, 83, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Preston, S.D.; de Waal, F.B. Empathy: Its ultimate and proximate bases. Behav. Brain Sci. 2002, 25, 1–20, discussion 20–71. [Google Scholar] [CrossRef] [Green Version]
- Shamay-Tsoory, S.G. The neural bases for empathy. Neuroscientist 2011, 17, 18–24. [Google Scholar] [CrossRef]
- Zaki, J.; Ochsner, K. The neuroscience of empathy: Progress, pitfalls and promise. Nat. Neurosci. 2012, 15, 675–680. [Google Scholar] [CrossRef]
- Decety, J.; Jackson, P.L. The functional architecture of human empathy. Behav. Cogn. Neurosci. Rev. 2004, 3, 71–100. [Google Scholar] [CrossRef]
- Innamorati, M.; Ebisch, S.J.H.; Gallese, V.; Saggino, A. A bidimensional measure of empathy: Empathic Experience Scale. PLoS ONE 2019, 14, e0216164. [Google Scholar] [CrossRef] [Green Version]
- Banissy, M.J.; Kanai, R.; Walsh, V.; Rees, G. Inter-individual differences in empathy are reflected in human brain structure. Neuroimage 2012, 62, 2034–2039. [Google Scholar] [CrossRef] [Green Version]
- Eres, R.; Decety, J.; Louis, W.R.; Molenberghs, P. Individual differences in local gray matter density are associated with differences in affective and cognitive empathy. Neuroimage 2015, 117, 305–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christov-Moore, L.; Reggente, N.; Douglas, P.K.; Feusner, J.D.; Iacoboni, M. Predicting Empathy From Resting State Brain Connectivity: A Multivariate Approach. Front. Integr. Neurosci. 2020, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Esménio, S.; Soares, J.M.; Oliveira-Silva, P.; Zeidman, P.; Razi, A.; Gonçalves, Ó.F.; Friston, K.; Coutinho, J. Using resting-state DMN effective connectivity to characterize the neurofunctional architecture of empathy. Sci. Rep. 2019, 9, 2603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winters, D.E.; Pruitt, P.J.; Fukui, S.; Cyders, M.A.; Pierce, B.J.; Lay, K.; Damoiseaux, J.S. Network functional connectivity underlying dissociable cognitive and affective components of empathy in adolescence. Neuropsychologia 2021, 156, 107832. [Google Scholar] [CrossRef]
- Cox, C.L.; Uddin, L.Q.; Di Martino, A.; Castellanos, F.X.; Milham, M.P.; Kelly, C. The balance between feeling and knowing, affective and cognitive empathy are reflected in the brain’s intrinsic functional dynamics. Soc. Cogn. Affect Neurosci. 2012, 201, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Friston, K. Structural and functional brain networks: From connections to cognition. Science 2013, 342, 1238411. [Google Scholar] [CrossRef] [Green Version]
- de Pasquale, F.; Corbetta, M.; Betti, V.; Della Penna, S. Cortical cores in network dynamics. Neuroimage 2018, 180, 370–382. [Google Scholar] [CrossRef]
- van den Heuvel, M.P.; Sporns, O. Network hubs in the human brain. Trends Cogn. Sci. 2013, 17, 683–696. [Google Scholar] [CrossRef]
- Alstott, J.; Breakspear, M.; Hagmann, P.; Cammoun, L.; Sporns, O. Modeling the impact of lesions in the human brain. PLoS Comput. Biol. 2009, e1000408. [Google Scholar] [CrossRef] [Green Version]
- Aerts, H.; Fias, W.; Caeyenberghs, K.; Marinazzo, D. Brain networks under attack, robustness properties and the impact of lesions. Brain 2016, 139, 3063–3083. [Google Scholar] [CrossRef]
- Gratton, C.; Nomura, E.M.; Pérez, F.; D’Esposito, M. Focal brain lesions to critical locations cause widespread disruption of the modular organization of the brain. J. Cogn. Neurosci. 2012, 24, 1275–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillis, A.E. Inability to empathize: Brain lesions that disrupt sharing and understanding another’s emotions. Brain 2014, 137, 981–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamay-Tsoory, S.G.; Aharon-Peretz, J.; Perry, D. Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 2009, 132, 617–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lough, S.; Kipps, C.M.; Treise, C.; Watson, P.; Blair, J.R.; Hodges, J.R. Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia 2006, 44, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Baez, S.; Manes, F.; Huepe, D.; Torralva, T.; Fiorentino, N.; Richter, F.; Huepe-Artigas, D.; Ferrari, J.; Montañes, P.; Reyes, P.; et al. Primary empathy deficits in frontotemporal dementia. Front. Aging Neurosci. 2014, 6, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hétu, S.; Taschereau-Dumouchel, V.; Jackson, P.L. Stimulating the brain to study social interactions and empathy. Brain Stimul. 2012, 5, 95–102. [Google Scholar] [CrossRef]
- Bukowski, H.; Tik, M.; Silani, G.; Ruff, C.C.; Windischberger, C.; Lamm, C. When differences matter: rTMS/fMRI reveals how differences in dispositional empathy translate to distinct neural underpinnings of self-other distinction in empathy. Cortex 2020, 128, 143–161. [Google Scholar] [CrossRef] [Green Version]
- Paradiso, E.; Gazzola, V.; Keysers, C. Neural mechanisms necessary for empathy-related phenomena across species. Curr. Opin. Neurobiol. 2021, 68, 107–115. [Google Scholar] [CrossRef]
- Stotland, E.T. Exploratory investigations of empathy. In Advances in Experimental Social Psychology; Berkowitz, L., Ed.; Academic Press: New York, NY, USA, 1969; pp. 271–314. [Google Scholar]
- Cottrell, L.S. Some neglected problems in social psychology. Am. Sociol. Rev. 1950, 15, 705–712. [Google Scholar] [CrossRef]
- Gallese, V. Before and below ‘theory of mind’: Embodied simulation and the neural correlates of social cognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 659–669. [Google Scholar] [CrossRef] [Green Version]
- Spaulding, S. Cognitive empathy. In The Routledge Handbook of Philosophy of Empathy; Routledge: London, UK, 2017; pp. 13–21. [Google Scholar]
- Davis, M.H. A multidimensional approach to individual differences in empathy. JSAS Cat. Sel. Doc. Psychol. 1980, 10, 85. [Google Scholar]
- Chrysikou, E.G.; Thompson, W.J. Assessing Cognitive and Affective Empathy Through the Interpersonal Reactivity Index: An Argument Against a Two-Factor Model. Assessment 2016, 23, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Scheler, M. The Nature of Sympathy; Transaction Publishers: Piscataway, NJ, USA, 2008. [Google Scholar]
- Singer, T.; Lamm, C. The social neuroscience of empathy. Ann. N. Y. Acad. Sci. 2009, 1156, 81–96. [Google Scholar] [CrossRef]
- Stam, C.J.; Reijneveld, J.C. Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed. Phys. 2007, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Borgatti, S.P. Centrality and Network Flow. Soc. Networks 2005, 27, 55–71. [Google Scholar] [CrossRef]
- Borgatti, S.P.; Everett, M.G. Graph-Theoretic Perspective on Centrality. Soc. Netw. 2006, 28, 466–484. [Google Scholar] [CrossRef]
- Friedkin, N.E. Theoretical foundations for centrality measures. Am. J. Sociol. 1991, 96, 1478–1504. [Google Scholar] [CrossRef]
- Christov-Moore, L.; Simpson, E.A.; Coudé, G.; Grigaityte, K.; Iacoboni, M.; Ferrari, P.F. Empathy: Gender effects in brain and behavior. Neurosci. Biobehav. Rev. 2014, 46, 604–627. [Google Scholar] [CrossRef] [Green Version]
- Schulte-Rüther, M.; Markowitsch, H.J.; Shah, N.J.; Fink, G.R.; Piefke, M. Gender differences in brain networks supporting empathy. Neuroimage 2008, 42, 393–403. [Google Scholar] [CrossRef]
- Gallese, V. Embodied simulation: From neurons to phenomenal experience. Phenomenol. Cogn. Sci. 2005, 4, 23–48. [Google Scholar] [CrossRef]
- Gallese, V. Bodily Selves in Relation: Embodied simulation as second-person perspective on intersubjectivity. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 20130177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molnar-Szakacs, I.; Uddin, L.Q. Self-processing and the default mode network: Interactions with the mirror neuron system. Front. Hum. Neurosci. 2013, 7, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Duncan, N.W.; de Greck, M.; Northoff, G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci. Biobehav. Rev. 2011, 35, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, B.C.; Singer, T. The neural basis of empathy. Annu. Rev. Neurosci. 2012, 35, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, P.; Northoff, G. How is our self related to midline regions and the default-mode network? Neuroimage 2011, 57, 1221–1233. [Google Scholar] [CrossRef]
- Di Plinio, S.; Ebisch, S.J.H. Brain network profiling defines functionally specialized cortical networks. Hum. Brain Mapp. 2018, 39, 4689–4706. [Google Scholar] [CrossRef] [Green Version]
- Uddin, L.Q.; Iacoboni, M.; Lange, C.; Keenan, J.P. The self and social cognition: The role of cortical midline structures and mirror neurons. Trends Cogn. Sci. 2007, 11, 153–157. [Google Scholar] [CrossRef]
- Northoff, G.; Panksepp, J. The trans-species concept of self and the subcortical-cortical midline system. Trends Cogn. Sci. 2008, 12, 259–264. [Google Scholar] [CrossRef]
- Keysers, C.; Gazzola, V. Expanding the mirror: Vicarious activity for actions, emotions, and sensations. Curr. Opin. Neurobiol. 2009, 19, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Gallese, V.; Sinigaglia, C. What is so special about embodied simulation? Trends Cogn. 2011, 15, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Fogassi, L.; Gallese, V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat. Rev. Neurosci. 2001, 2, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Molenberghs, P.; Cunnington, R.; Mattingley, J.B. Brain regions with mirror properties: A meta-analysis of 125 human fMRI studies. Neurosci. Biobehav. Rev. 2012, 36, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Perry, A.; Saunders, S.N.; Stiso, J.; Dewar, C.; Lubell, J.; Meling, T.R.; Solbakk, A.K.; Endestad, T.; Knight, R.T. Effects of prefrontal cortex damage on emotion understanding: EEG and behavioural evidence. Brain 2017, 140, 1086–1099. [Google Scholar] [CrossRef]
- Eslinger, P.J.; Moore, P.; Anderson, C.; Grossman, M. Social cognition, executive functioning, and neuroimaging correlates of empathic deficits in frontotemporal dementia. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Bell, P.T.; Shine, J.M. Subcortical contributions to large-scale network communication. Neurosci. Biobehav. Rev. 2016, 71, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Oldham, S.; Fornito, A. The development of brain network hubs. Dev. Cogn. Neurosci. 2019, 36, 100607. [Google Scholar] [CrossRef]
- Decety, J.; Norman, G.J.; Berntson, G.G.; Cacioppo, J.T. A neurobehavioral evolutionary perspective on the mechanisms underlying empathy. Prog. Neurobiol. 2012, 98, 38–48. [Google Scholar] [CrossRef]
- Couto, B.; Sedeño, L.; Sposato, L.A.; Sigman, M.; Riccio, P.M.; Salles, A.; Lopez, V.; Schroeder, J.; Manes, F.; Ibanez, A. Insular networks for emotional processing and social cognition: Comparison of two case reports with either cortical or subcortical involvement. Cortex 2013, 49, 1420–1434. [Google Scholar] [CrossRef]
- Weddell, R.A. Effects of subcortical lesion site on human emotional behavior. Brain Cogn. 1994, 25, 161–193. [Google Scholar] [CrossRef] [PubMed]
- Masterman, D.L.; Cummings, J.L. Frontal-subcortical circuits: The anatomic basis of executive, social and motivated behaviors. J. Psychopharmacol. 1997, 11, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Di Plinio, S.; Perrucci, M.G.; Ebisch, S.J.H. The Prospective Sense of Agency is Rooted in Local and Global Properties of Intrinsic Functional Brain Networks. J. Cogn. Neurosci. 2020, 32, 1764–1779. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.W. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996, 9, 162–173. [Google Scholar] [CrossRef]
- Chen, G.; Taylor, P.A.; Cox, R.W. Is the statistic value all we should care about in neuroimaging? Neuroimage 2017, 147, 952–959. [Google Scholar] [CrossRef]
- Power, J.D.; Mitra, A.; Laumann, T.O.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 2014, 84, 320–341. [Google Scholar] [CrossRef]
- Caballero-Gaudes, C.; Reynolds, R.C. Methods for cleaning the BOLD fMRI signal. Neuroimage 2017, 154, 128–149. [Google Scholar] [CrossRef] [Green Version]
- Ciric, R.; Rosen, A.F.G.; Erus, G.; Cieslak, M.; Adebimpe, A.; Cook, P.A.; Bassett, D.S.; Davatzikos, C.; Wolf, D.H.; Satterthwaite, T.D. Mitigating head motion artifact in functional connectivity MRI. Nat. Protoc. 2018, 13, 2801–2826. [Google Scholar] [CrossRef]
- Weissenbacher, A.; Kasess, C.; Gerstl, F.; Lanzenberger, R.; Moser, E.; Windischberger, C. Correlations and anticorrelations in resting-state functional connectivity MRI: A quantitative comparison of preprocessing strategies. Neuroimage 2009, 47, 1408–1416. [Google Scholar] [CrossRef]
- Saad, Z.S.; Gotts, S.J.; Murphy, K.; Chen, G.; Jo, H.J.; Martin, A.; Cox, R.W. Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012, 2, 25–32. [Google Scholar] [CrossRef]
- Joliot, M.; Jobard, G.; Naveau, M.; Delcroix, N.; Petit, L.; Zago, L.; Crivello, F.; Mellet, E.; Mazoyer, B.; Tzourio-Mazoyer, N. AICHA: An atlas of intrinsic connectivity of homotopic areas. J. Neurosci. Methods 2015, 254, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Diedrichsen, J.; Balsters, J.H.; Flavell, J.; Cussans, E.; Ramnani, N. A probabilistic MR atlas of the human cerebellum. Neuroimage 2009, 46, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 2010, 52, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef] [Green Version]
- Achard, S.; Bullmore, E. Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 2007, 3, e17. [Google Scholar] [CrossRef]
- van den Heuvel, M.P.; de Lange, S.C.; Zalesky, A.; Seguin, C.; Yeo, B.T.T.; Schmidt, R. Proportional thresholding in resting-state fMRI functional connectivity networks and consequences for patient-control connectome studies: Issues and recommendations. NeuroImage 2017, 152, 437–449. [Google Scholar] [CrossRef]
- Van den Heuvel, M.P.; Stam, C.J.; Boersma, M.; Pol, H.E.H. Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. Neuroimage 2008, 43, 11. [Google Scholar] [CrossRef]
- Ginestet, C.E.; Nichols, T.E.; Bullmore, E.T.; Simmons, A. Brain network analysis: Separating cost from topology using cost-integration. PLoS ONE 2011, 6, e21570. [Google Scholar] [CrossRef]
- Porter, M.A.; Onnela, J.-P.; Mucha, P.J. Communities in networks. Not. Am. Math. Soc. 2009, 56, 1082–1097. [Google Scholar]
- Blondel, V.D.; Guillaume, J.L.; Hendrickx, J.M.; de Kerchove, C.; Lambiotte, R. Local leaders in random networks. Phys. Rev. E Stat. Nonlin. Soft Matter. Phys. 2008, 77, 036114. [Google Scholar] [CrossRef] [Green Version]
- Lancichinetti, A.; Fortunato, S. Benchmarks for testing community detection algorithms on directed and weighted graphs with overlapping communities. Phys. Rev. E Stat. Nonlin. Soft Matter. Phys. 2009, 80, 016118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Bagrow, J.P.; Bollt, E.M.; Skufca, J.D. Dynamic computation of network statistics via updating schema. Phys. Rev. E Stat. Nonlin. Soft Matter. Phys. 2009, 79, 036116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassett, D.S.; Wymbs, N.F.; Porter, M.A.; Mucha, P.J.; Carlson, J.M.; Grafton, S.T. Dynamic reconfiguration of human brain networks during learning. Proc. Natl. Acad. Sci. USA 2011, 108, 7641–7646. [Google Scholar] [CrossRef] [Green Version]
- Lancichinetti, A.; Fortunato, S. Consensus clustering in complex networks. Sci Rep 2012, 2, 336. [Google Scholar] [CrossRef] [PubMed]
- Betzel, R.F.; Bassett, D.S. Multi-scale brain networks. Neuroimage 2017, 160, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, M.E.J.; Girvan, M. Finding and evaluating community structure in networks. Phys. Rev. E 2004, 69, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortunato, S.; Barthélemy, M. Resolution limit in community detection. Proc. Natl. Acad. Sci. USA 2007, 104, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Guimerà, R.; Nunes Amaral, L.A. Functional cartography of complex metabolic networks. Nature 2005, 433, 895–900. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Q.; Rong, J.Y.; Liu, X.Y. Best linear unbiased prediction for linear combinations in general mixed linear models. J. Multivar. Anal. 2008, 99, 1503–1517. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, A.; Kapelner, A.; Bleich, J.; Pitkin, E. Peeking Inside the Black Box: Visualizing Statistical Learning With Plots of Individual Conditional Expectation. J. Comput. Graph. Stat. 2015, 24, 44–65. [Google Scholar] [CrossRef]
- Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.L.; De La Vega, A.; Mills, C.; Andrews-Hanna, J.; Spreng, R.N.; Cole, M.W.; Christoff, K. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc. Natl. Acad. Sci. USA 2018, 115, E1598–E1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marek, S.; Dosenbach, N.U.F. The fronto-parietal network: Function, electrophysiology, and importance of individual precision mapping. Dialogues Clin. Neurosci. 2018, 20, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Zanto, T.P.; Gazzaley, A. Fronto-parietal network: Flexible hub of cognitive control. Trends Cogn. Sci. 2013, 17, 602–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzolatti, G.; Sinigaglia, C. The functional role of the parieto-frontal mirror circuit: Interpretations and misinterpretations. Nat. Rev. Neurosci. 2010, 11, 264–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozzi, S. and Fogassi, L. Neural Coding for Action Execution and Action Observation in the Prefrontal Cortex and Its Role in the Organization of Socially Driven Behavior. Front. Neurosci. 2017, 11, 492. [Google Scholar] [CrossRef]
- Shamay-Tsoory, S.G.; Tomer, R.; Berger, B.D.; Aharon-Peretz, J. Characterization of empathy deficits following prefrontal brain damage: The role of the right ventromedial prefrontal cortex. J. Cogn. Neurosci. 2003, 15, 324–337. [Google Scholar] [CrossRef]
- Li, W.; Yang, P.; Ngetich, R.K.; Zhang, J.; Jin, Z.; Li, L. Differential involvement of fronto-parietal network and insula cortex in emotion regulation. Neuropsychologia 2021, 161, 107991. [Google Scholar] [CrossRef]
- Northoff, G.; Qin, P.; Nakao, T. Rest-stimulus interaction in the brain: A review. Trends Neurosci. 2010, 33, 277–284. [Google Scholar] [CrossRef]
- Northoff, G.; Sibille, E. Why are cortical GABA neurons relevant to internal focus in depression? A cross-level model linking cellular, biochemical and neural network findings. Mol. Psychiatry 2014, 19, 966–977. [Google Scholar] [CrossRef] [Green Version]
- Ebisch, S.J.; Ferri, F.; Salone, A.; Perrucci, M.G.; D’Amico, L.; Ferro, F.M.; Romani, G.L.; Gallese, V. Differential involvement of somatosensory and interoceptive cortices during the observation of affective touch. J. Cogn. Neurosci. 2011, 23, 1808–1822. [Google Scholar] [CrossRef] [PubMed]
- Burkett, J.P.; Spiegel, L.L.; Inoue, K.; Murphy, A.Z.; Young, L.J. Activation of μ-opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology 2011, 36, 2200–2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balleine, B.W.; Delgado, M.R.; Hikosaka, O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007, 27, 8161–8165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, J.C.; Dunne, S.; Furey, T.; O’Doherty, J.P. Human dorsal striatum encodes prediction errors during observational learning of instrumental actions. J. Cogn. Neurosci. 2012, 24, 106–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rilling, J.; Gutman, D.; Zeh, T.; Pagnoni, G.; Berns, G.; Kilts, C. A neural basis for social cooperation. Neuron 2002, 35, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Viskontas, I.V.; Possin, K.L.; Miller, B.L. Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Ann. N. Y. Acad. Sci. 2007, 1121, 528–545. [Google Scholar] [CrossRef] [Green Version]
- Keysers, C.; Kaas, J.H.; Gazzola, V. Somatosensation in social perception. Nat. Rev. Neurosci. 2010, 11, 417–428. [Google Scholar] [CrossRef]
- Schaefer, M.; Kühnel, A.; Rumpel, F.; Gärtner, M. Altruistic Acting Caused by a Touching Hand, Neural Underpinnings of the Midas Touch Effect. Soc. Cogn. Affect Neurosci. 2021, 00, 1–10. [Google Scholar] [CrossRef]
- Carr, L.; Iacoboni, M.; Dubeau, M.C.; Mazziotta, J.C.; Lenzi, G.L. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proc. Natl. Acad. Sci. USA 2003, 100, 5497–5502. [Google Scholar] [CrossRef] [Green Version]
- Hooker, C.I.; Verosky, S.C.; Germine, L.T.; Knight, R.T.; D’Esposito, M. Neural activity during social signal perception correlates with self-reported empathy. Brain Res. 2010, 1308, 100–113. [Google Scholar] [CrossRef] [Green Version]
- Avenanti, A.; Bueti, D.; Galati, G.; Aglioti, S.M. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat. Neurosci. 2005, 8, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Arioli, M.; Cattaneo, Z.; Ricciardi, E.; Canessa, N. Overlapping and specific neural correlates for empathizing, affective mentalizing, and cognitive mentalizing: A coordinate-based meta-analytic study. Hum. Brain Mapp. 2021, 42, 4777–4804. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. How do you feel? Interoception, the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002, 3, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Wiens, S.; Rotshtein, P.; Ohman, A.; Dolan, R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004, 7, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Scalabrini, A.; Ebisch, S.J.; Huang, Z.; Di Plinio, S.; Perrucci, M.G.; Romani, G.L.; Mucci, C.; Northoff, G. Spontaneous brain activity predicts task-evoked activity during animate versus inanimate touch. Cerebral Cortex 2019, 29, 4628–4645. [Google Scholar] [CrossRef] [Green Version]
- Scalabrini, A.; Huang, Z.; Mucci, C.; Perrucci, M.G.; Ferretti, A.; Fossati, A.; Romani, G.L.; Northoff, G.; Ebisch, S.J. How spontaneous brain activity and narcissistic features shape social interaction. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Uddin, L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015, 16, 55–61. [Google Scholar] [CrossRef]
- De Pasquale, F.; Sabatini, U.; Della Penna, S.; Sestieri, C.; Caravasso, C.F.; Formisano, R.; Péran, P. The connectivity of functional cores reveals different degrees of segregation and integration in the brain at rest. Neuroimage 2013, 69, 51–61. [Google Scholar] [CrossRef]
- Sridharan, D.; Levitin, D.J.; Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. USA 2008, 105, 12569–12574. [Google Scholar] [CrossRef] [Green Version]
- Kanske, P.; Böckler, A.; Singer, T. Models, Mechanisms and Moderators: Dissociating Empathy and Theory of Mind. Curr. Top Behav. Neurosci. 2017, 30, 193–206. [Google Scholar] [CrossRef]
- Thioux, M.; Keysers, C. Empathy: Shared circuits and their dysfunctions. Dialogues Clin. Neurosci. 2010, 12, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Bolt, T.; Nomi, J.S.; Li, J.; Zheng, X.; Fu, M.; Kendrick, K.M.; Becker, B.; Uddin, L.Q. Inter-subject phase synchronization differentiates neural networks underlying physical pain empathy. Soc. Cogn. Affect Neurosci. 2020, 15, 225–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, S.; Becker, B.; Geng, Y.; Zhao, Z.; Xu, X.; Zhao, W.; Ren, P.; Kendrick, K.M. Voluntary control of anterior insula and its functional connections is feedback-independent and increases pain empathy. Neuroimage 2016, 130, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Bird, G.; Silani, G.; Brindley, R.; White, S.; Frith, U.; Singer, T. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain 2010, 133, 1515–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogeveen, J.; Bird, G.; Chau, A.; Krueger, F.; Grafman, J. Acquired alexithymia following damage to the anterior insula. Neuropsychologia 2016, 82, 142–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamm, C.; Decety, J.; Singer, T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 2011, 54, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Wang, M.; Northoff, G. Linking Bodily, Environmental and Mental States in the Self—A Three-Level Model Based on a Meta-Analysis. Neurosci. Biobehav. Rev. 2020, 115, 77–95. [Google Scholar] [CrossRef]
- Scalabrini, A.; Wolman, A.; Northoff, G. The self and its right insula—Differential topography and dynamic of right vs. left insula. Brain Sci. 2021, 11, 1312. [Google Scholar] [CrossRef]
- Gallese, V.; Keysers, C.; Rizzolatti, G. A unifying view of the basis of social cognition. Trends Cogn. Sci. 2004, 8, 396–403. [Google Scholar] [CrossRef]
- Chau, A.; Zhong, W.; Gordon, B.; Krueger, F.; Grafman, J. Anterior insula lesions and alexithymia reduce the endorsements of everyday altruistic attitudes. Neuropsychologia 2018, 117, 428–439. [Google Scholar] [CrossRef]
- Belfi, A.M.; Koscik, T.R.; Tranel, D. Damage to the insula is associated with abnormal interpersonal trust. Neuropsychologia 2015, 71, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadland, K.A.; Rushworth, M.S.F.; Gaffan, D.; Passingham, R.E. The effect of cingulate lesions on social behaviour and emotion. Neuropsychologia 2003, 41, 919–931. [Google Scholar] [CrossRef]
- Lennon, R.; Eisenberg, N. Emotional displays associated with preschoolers’ prosocial behavior. Child Dev. 1987, 58, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Michalska, K.J.; Kinzler, K.D.; Decety, J. Age-related sex differences in explicit measures of empathy do not predict brain responses across childhood and adolescence. Dev. Cogn. Neurosci. 2013, 3, 22–32. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, E.; Konrath, S.H.; Grühn, D.; Hagen, A.L. Empathic concern and perspective taking: Linear and quadratic effects of age across the adult life span. J. Gerontol. B Psychol. Sci. Soc. Sci. 2013, 68, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Bembich, S.; Saksida, A.; Mastromarino, S.; Travan, L.; Di Risio, G.; Cont, G.; Demarini, S. Empathy at birth: Mother’s cortex synchronizes with that of her newborn in pain. Eur. J. Neurosci. 2022, 55, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Trevarthen, C. Communication and cooperation in early infancy:A description of primary intersubjectivity. In Before Speech; Bullowa, M., Ed.; Cambridge University Press: Cambridge, UK, 1979. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebisch, S.J.H.; Scalabrini, A.; Northoff, G.; Mucci, C.; Sergi, M.R.; Saggino, A.; Aquino, A.; Alparone, F.R.; Perrucci, M.G.; Gallese, V.; et al. Intrinsic Shapes of Empathy: Functional Brain Network Topology Encodes Intersubjective Experience and Awareness Traits. Brain Sci. 2022, 12, 477. https://doi.org/10.3390/brainsci12040477
Ebisch SJH, Scalabrini A, Northoff G, Mucci C, Sergi MR, Saggino A, Aquino A, Alparone FR, Perrucci MG, Gallese V, et al. Intrinsic Shapes of Empathy: Functional Brain Network Topology Encodes Intersubjective Experience and Awareness Traits. Brain Sciences. 2022; 12(4):477. https://doi.org/10.3390/brainsci12040477
Chicago/Turabian StyleEbisch, Sjoerd J. H., Andrea Scalabrini, Georg Northoff, Clara Mucci, Maria Rita Sergi, Aristide Saggino, Antonio Aquino, Francesca R. Alparone, Mauro Gianni Perrucci, Vittorio Gallese, and et al. 2022. "Intrinsic Shapes of Empathy: Functional Brain Network Topology Encodes Intersubjective Experience and Awareness Traits" Brain Sciences 12, no. 4: 477. https://doi.org/10.3390/brainsci12040477
APA StyleEbisch, S. J. H., Scalabrini, A., Northoff, G., Mucci, C., Sergi, M. R., Saggino, A., Aquino, A., Alparone, F. R., Perrucci, M. G., Gallese, V., & Di Plinio, S. (2022). Intrinsic Shapes of Empathy: Functional Brain Network Topology Encodes Intersubjective Experience and Awareness Traits. Brain Sciences, 12(4), 477. https://doi.org/10.3390/brainsci12040477