Cognitive Deficits among Individuals Admitted to a Post-Acute Pneumological Rehabilitation Unit in Southern Italy after COVID-19 Infection
Abstract
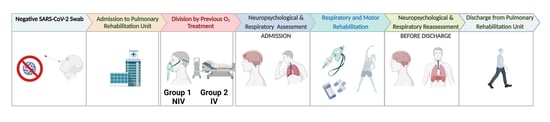
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Neuropsychological Data
2.3. Pneumological and Motor Evaluation
2.4. Statistical Analysis
3. Results
3.1. Descriptive Statistics for Patient Groups
3.2. Neuropsychological Measures
3.3. Pneumological and Motor Measures
3.4. Examination at Discharge, One Month Later
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solomon, T. Neurological Infection with SARS-CoV-2—The Story so Far. Nat. Rev. Neurol. 2021, 17, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, K.; Mahanty, S. Respiratory Virus Infections: Understanding COVID-19. Immunity 2020, 52, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Al-Ramadan, A.; Rabab’h, O.; Shah, J.; Gharaibeh, A. Acute and Post-Acute Neurological Complications of COVID-19. Neurol. Int. 2021, 13, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, S.R.; Panzeri, A.; Cerutti, P.; Sacco, D. The Psychological Experience and Intervention in Post-Acute COVID-19 Inpatients. Neuropsychiatr. Dis. Treat 2021, 17, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, F.; Prasad, K.; Kumar, V. Neurological Manifestations of COVID-19: Available Evidences and a New Paradigm. J. Neurovirol. 2020, 26, 619–630. [Google Scholar] [CrossRef]
- Miners, S.; Kehoe, P.G.; Love, S. Cognitive Impact of COVID-19: Looking beyond the Short Term. Alzheimer’s Res. Ther. 2020, 12, 170. [Google Scholar] [CrossRef]
- Steardo, L.; Steardo, L.; Verkhratsky, A. Psychiatric Face of COVID-19. Transl. Psychiatry 2020, 10, 261. [Google Scholar] [CrossRef]
- Almeria, M.; Cejudo, J.C.; Sotoca, J.; Deus, J.; Krupinski, J. Cognitive Profile Following COVID-19 Infection: Clinical Predictors Leading to Neuropsychological Impairment. Brain Behav. Immun. Health 2020, 9, 100163. [Google Scholar] [CrossRef]
- Llach, C.D.; Vieta, E. Mind Long COVID: Psychiatric Sequelae of SARS-CoV-2 Infection. Eur. Neuropsychopharmacol. 2021, 49, 119–121. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and Neuropsychiatric Presentations Associated with Severe Coronavirus Infections: A Systematic Review and Meta-Analysis with Comparison to the COVID-19 Pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Monti, G.; Leggieri, C.; Fominskiy, E.; Scandroglio, A.M.; Colombo, S.; Tozzi, M.; Moizo, E.; Mucci, M.; Crivellari, M.; Pieri, M.; et al. Two-Months Quality of Life of COVID-19 Invasively Ventilated Survivors; an Italian Single-Center Study. Acta Anaesthesiol. Scand. 2021, 65, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Jaywant, A.; Vanderlind, W.M.; Alexopoulos, G.S.; Fridman, C.B.; Perlis, R.H.; Gunning, F.M. Frequency and Profile of Objective Cognitive Deficits in Hospitalized Patients Recovering from COVID-19. Neuropsychopharmacology 2021, 46, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, R.; Dini, M.; Groppo, E.; Rosci, C.; Reitano, M.R.; Bai, F.; Poletti, B.; Brugnera, A.; Silani, V.; Monforte, A.D.; et al. Long-Lasting Cognitive Abnormalities after COVID-19. Brain Sci. 2021, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Miskowiak, K.W.; Johnsen, S.; Sattler, S.M.; Nielsen, S.; Kunalan, K.; Rungby, J.; Lapperre, T.; Porsberg, C.M. Cognitive Impairments Four Months after COVID-19 Hospital Discharge: Pattern, Severity and Association with Illness Variables. Eur. Neuropsychopharmacol. 2021, 46, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Tavares-Júnior, J.W.L.; de Souza, A.C.C.; Borges, J.W.P.; Oliveira, D.N.; Siqueira-Neto, J.I.; Sobreira-Neto, M.A.; Braga-Neto, P. COVID-19 Associated Cognitive Impairment: A Systematic Review. Cortex 2022, 152, 77–97. [Google Scholar] [CrossRef]
- Hadad, R.; Khoury, J.; Stanger, C.; Fisher, T.; Schneer, S.; Ben-Hayun, R.; Possin, K.; Valcour, V.; Aharon-Peretz, J.; Adir, Y. Cognitive Dysfunction Following COVID-19 Infection. J. Neurovirol. 2022, 28, 430–437. [Google Scholar] [CrossRef]
- Sartori, A.C.; Vance, D.E.; Slater, L.Z.; Crowe, M. The Impact of Inflammation on Cognitive Function in Older Adults: Implications for Healthcare Practice and Research. J. Neurosci. Nurs. 2012, 44, 206–217. [Google Scholar] [CrossRef] [Green Version]
- Warren-Gash, C.; Forbes, H.J.; Williamson, E.; Breuer, J.; Hayward, A.C.; Mavrodaris, A.; Ridha, B.H.; Rossor, M.N.; Thomas, S.L.; Smeeth, L. Human Herpesvirus Infections and Dementia or Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 4743. [Google Scholar] [CrossRef] [Green Version]
- Antonelli-Incalzi, R.; Corsonello, A.; Trojano, L.; Acanfora, D.; Spada, A.; Izzo, O.; Rengo, F. Correlation between Cognitive Impairment and Dependence in Hypoxemic COPD. J. Clin. Exp. Neuropsychol. 2008, 30, 141–150. [Google Scholar] [CrossRef]
- Herridge, M.S.; Moss, M.; Hough, C.L.; Hopkins, R.O.; Rice, T.W.; Bienvenu, O.J.; Azoulay, E. Recovery and Outcomes after the Acute Respiratory Distress Syndrome (ARDS) in Patients and Their Family Caregivers. Intensive Care Med. 2016, 42, 725–738. [Google Scholar] [CrossRef]
- Wang, J.; Song, R.; Dove, A.; Qi, X.; Ma, J.; Laukka, E.J.; Bennett, D.A.; Xu, W. Pulmonary Function Is Associated with Cognitive Decline and Structural Brain Differences. Alzheimer’s Dement. 2022, 18, 1335–1344. [Google Scholar] [CrossRef]
- Vitacca, M.; Carone, M.; Clini, E.M.; Paneroni, M.; Lazzeri, M.; Lanza, A.; Privitera, E.; Pasqua, F.; Gigliotti, F.; Castellana, G.; et al. Joint Statement on the Role of Respiratory Rehabilitation in the COVID-19 Crisis: The Italian Position Paper. Respiration 2020, 99, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Arenivas, A.; Carter, K.R.; Harik, L.M.; Hays, K.M. COVID-19 Neuropsychological Factors and Considerations within the Acute Physical Medicine and Rehabilitation Setting. Brain Inj. 2020, 34, 1136–1137. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.; Robinson, L. Rehabilitation after Critical Illness in People with COVID-19 Infection. Am. J. Phys. Med. Rehabil. 2020, 99, 470–474. [Google Scholar] [CrossRef]
- Alemanno, F.; Houdayer, E.; Parma, A.; Spina, A.; del Forno, A.; Scatolini, A.; Angelone, S.; Brugliera, L.; Tettamanti, A.; Beretta, L.; et al. COVID-19 Cognitive Deficits after Respiratory Assistance in the Subacute Phase: A COVID Rehabilitation Unit Experience. PLoS One 2021, 16, e0246590. [Google Scholar] [CrossRef] [PubMed]
- Manera, M.R.; Fiabane, E.; Pain, D.; Aiello, E.N.; Radici, A.; Ottonello, M.; Padovani, M.; Wilson, B.A.; Fish, J.; Pistarini, C. Clinical Features and Cognitive Sequelae in COVID-19: A Retrospective Study on N=152 Patients. Neurol. Sci. 2022, 43, 45–50. [Google Scholar] [CrossRef]
- Woo, M.S.; Malsy, J.; Pöttgen, J.; Seddiq Zai, S.; Ufer, F.; Hadjilaou, A.; Schmiedel, S.; Addo, M.M.; Gerloff, C.; Heesen, C.; et al. Frequent Neurocognitive Deficits after Recovery from Mild COVID-19. Brain Commun. 2020, 2, fcaa205. [Google Scholar] [CrossRef]
- Fotuhi, M.; Mian, A.; Meysami, S.; Raji, C.A. Neurobiology of COVID-19. J. Alzheimer’s Dis. 2020, 76, 3–19. [Google Scholar] [CrossRef]
- Holmes, E.A.; O’Connor, R.C.; Perry, V.H.; Tracey, I.; Wessely, S.; Arseneault, L.; Ballard, C.; Christensen, H.; Cohen Silver, R.; Everall, I.; et al. Multidisciplinary Research Priorities for the COVID-19 Pandemic: A Call for Action for Mental Health Science. Lancet Psychiatry 2020, 7, 547–560. [Google Scholar] [CrossRef]
- Alnefeesi, Y.; Siegel, A.; Lui, L.M.W.; Teopiz, K.M.; Ho, R.C.M.; Lee, Y.; Nasri, F.; Gill, H.; Lin, K.; Cao, B.; et al. Impact of SARS-CoV-2 Infection on Cognitive Function: A Systematic Review. Front. Psychiatry 2021, 11, 621773. [Google Scholar] [CrossRef]
- Measso, G.; Cavarzeran, F.; Zappala, G.; Lebowitz, B.D.; Crook, T.H.; Pirozzolo, F.J.; Amaducci, L.A.; Massari Fidia SpA, D.; Terme, A.; Francesco Grigoletto, I. The Mini-Mental State Examination: Normative Study of an Italian Random Sample. Dev. Neuropsychol. 1993, 9, 77–85. [Google Scholar] [CrossRef]
- Appollonio, I.; Leone, M.; Isella, V.; Piamarta, F.; Consoli, T.; Villa, M.L.; Forapani, E.; Russo, A.; Nichelli, P. The Frontal Assessment Battery (FAB): Normative Values in an Italian Population Sample. Neurol. Sci. 2005, 26, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Ponteri, M.; Pioli, R.; Padovani, A.; Tunesi, S.; de Girolamo, G. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Adattamento Italiano; Giunti O.S.: Florence, Italy, 2007. [Google Scholar]
- Schoenberg, M.R.; Rinehardt, E.; Duff, K.; Mattingly, M.; Bharucha, K.J.; Scott, J.G. Assessing Reliable Change Using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) for Patients with Parkinson’s Disease Undergoing Deep Brain Stimulation (DBS) Surgery. Clin. Neuropsychol. 2012, 26, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Iani, L.; Lauriola, M.; Costantini, M. A Confirmatory Bifactor Analysis of the Hospital Anxiety and Depression Scale in an Italian Community Sample. Health Qual. Life Outcomes 2014, 12, 84. [Google Scholar] [CrossRef] [Green Version]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; Macintyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS Standards for Single-Breath Carbon Monoxide Uptake in the Lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef] [Green Version]
- Graham, B.L.; Steenbruggen, I.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; Miller, M.R.; et al. Standardization of Spirometry 2019 Update an Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, E70–E88. [Google Scholar] [CrossRef]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A.; van der Grinten, C.P.M.; Gustafsson, P.; Hankinson, J.; et al. Interpretative Strategies for Lung Function Tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.; Cooper, B. Improving the Sensitivity of the Barthel Index for Stroke Rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An Official European Respiratory Society/American Thoracic Society Technical Standard: Field Walking Tests in Chronic Respiratory Disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. American Thoracic Society ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Enright, P.L.; Sherrill, D.L. Reference Equations for the Six-Minute Walk in Healthy Adults. Am. J. Respir. Crit. Care Med. 1998, 158, 1384–1387. Available online: www.atsjournals.org (accessed on 15 June 2020). [CrossRef] [PubMed] [Green Version]
- Lacomis, D. The Use of Percutaneous Needle Muscle Biopsy in the Diagnosis of Myopathy. Curr. Rheumatol. Rep. 2000, 2, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar]
- Glickman, M.E.; Rao, S.R.; Schultz, M.R. False Discovery Rate Control Is a Recommended Alternative to Bonferroni-Type Adjustments in Health Studies. J. Clin. Epidemiol. 2014, 67, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Genovese, C.; Wasserman, L. Operating Characteristics and Extensions of the False Discovery Rate Procedure. J. R. Stat. Soc. Ser. B 2002, 64, 499–517. [Google Scholar]
- Moretta, P.; Ambrosino, P.; Lanzillo, A.; Marcuccio, L.; Fuschillo, S.; Papa, A.; Santangelo, G.; Trojano, L.; Maniscalco, M. Cognitive Impairment in Convalescent COVID-19 Patients Undergoing Multidisciplinary Rehabilitation: The Association with the Clinical and Functional Status. Healthcare 2022, 10, 480. [Google Scholar] [CrossRef]
- di Pietro, D.A.; Comini, L.; Gazzi, L.; Luisa, A.; Vitacca, M. Neuropsychological Pattern in a Series of Post-Acute COVID-19 Patients in a Rehabilitation Unit: Retrospective Analysis and Correlation with Functional Outcomes. Int. J. Environ. Res. Public Health 2021, 18, 5917. [Google Scholar] [CrossRef]
- Pistarini, C.; Fiabane, E.; Houdayer, E.; Vassallo, C.; Manera, M.R.; Alemanno, F. Cognitive and Emotional Disturbances Due to COVID-19: An Exploratory Study in the Rehabilitation Setting. Front. Neurol. 2021, 12, 643646. [Google Scholar] [CrossRef]
- Paneroni, M.; Vogiatzis, I.; Bertacchini, L.; Simonelli, C.; Vitacca, M. Predictors of Low Physical Function in Patients With COVID-19 With Acute Respiratory Failure Admitted to a Subacute Unit. Arch. Phys. Med. Rehabil. 2021, 102, 1228–1231. [Google Scholar] [CrossRef]
- Ortelli, P.; Ferrazzoli, D.; Sebastianelli, L.; Engl, M.; Romanello, R.; Nardone, R.; Bonini, I.; Koch, G.; Saltuari, L.; Quartarone, A.; et al. Neuropsychological and Neurophysiological Correlates of Fatigue in Post-Acute Patients with Neurological Manifestations of COVID-19: Insights into a Challenging Symptom. J. Neurol. Sci. 2021, 420, 117271. [Google Scholar] [CrossRef]
- Daroische, R.; Hemminghyth, M.S.; Eilertsen, T.H.; Breitve, M.H.; Chwiszczuk, L.J. Cognitive Impairment After COVID-19—A Review on Objective Test Data. Front. Neurol. 2021, 12, 699582. [Google Scholar] [CrossRef] [PubMed]
- Hampshire, A.; Trender, W.; Chamberlain, S.R.; Jolly, A.E.; Grant, J.E.; Patrick, F.; Mazibuko, N.; Williams, S.C.; Barnby, J.M.; Hellyer, P.; et al. Cognitive Deficits in People Who Have Recovered from COVID-19. EClinicalMedicine 2021, 39, 101044. [Google Scholar] [CrossRef] [PubMed]
- Sasannejad, C.; Ely, E.W.; Lahiri, S. Long-Term Cognitive Impairment after Acute Respiratory Distress Syndrome: A Review of Clinical Impact and Pathophysiological Mechanisms. Crit. Care 2019, 23, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellgren, L.; Birberg Thornberg, U.; Samuelsson, K.; Levi, R.; Divanoglou, A.; Blystad, I. Brain MRI and Neuropsychological Findings at Long-Term Follow-up after COVID-19 Hospitalisation: An Observational Cohort Study. BMJ Open 2021, 11, e055164. [Google Scholar] [CrossRef]
- Wahlgren, C.; Divanoglou, A.; Larsson, M.; Nilsson, E.; Balkhedb, Å.Ö.; Niward, K.; Thornberg, U.B.; Gudmundsson, E.L.; Levi, R. Rehabilitation Needs Following COVID-19: Five-Month Post-Discharge Clinical Follow-up of Individuals with Concerning Self-Reported Symptoms. EClinicalMedicine 2022, 43, 101219. [Google Scholar] [CrossRef]
- Elizabeth Wilcox, M.; Brummel, N.E.; Archer, K.; Wesley Ely, E.; Jackson, J.C.; Hopkins, R.O. Cognitive Dysfunction in ICU Patients: Risk Factors, Predictors, and Rehabilitation Interventions. Crit. Care Med. 2013, 41, S81–S98. [Google Scholar] [CrossRef]
- Blazhenets, G.; Schroeter, N.; Bormann, T.; Thurow, J.; Wagner, D.; Frings, L.; Weiller, C.; Meyer, P.T.; Dressing, A.; Hosp, J.A. Slow but Evident Recovery from Neocortical Dysfunction and Cognitive Impairment in a Series of Chronic COVID-19 Patients. J. Nucl. Med. 2021, 62, 910–915. [Google Scholar] [CrossRef]
- Zampogna, E.; Paneroni, M.; Belli, S.; Aliani, M.; Gandolfo, A.; Visca, D.; Bellanti, M.T.; Ambrosino, N.; Vitacca, M. Pulmonary Rehabilitation in Patients Recovering from COVID-19. Respiration 2021, 100, 416–422. [Google Scholar] [CrossRef]
- Daynes, E.; Gerlis, C.; Chaplin, E.; Gardiner, N.; Singh, S.J. Early Experiences of Rehabilitation for Individuals Post-COVID to Improve Fatigue, Breathlessness Exercise Capacity and Cognition–A Cohort Study. Chron. Respir. Dis. 2021, 18, 14799731211015691. [Google Scholar] [CrossRef]
- Patil, M.; Gupta, A.; Khanna, M.; Taly, A.B.; Soni, A.; Keshav Kumar, J.; Thennarasu, K. Cognitive and Functional Outcomes Following Inpatient Rehabilitation in Patients with Acquired Brain Injury: A Prospective Follow-up Study. J. Neurosci. Rural. Pract. 2017, 8, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Zhou, F.; Hou, W.; Silver, Z.; Wong, C.Y.; Chang, O.; Huang, E.; Zuo, Q.K. The Prevalence of Depression, Anxiety, and Sleep Disturbances in COVID-19 Patients: A Meta-Analysis. Ann. N. Y. Acad. Sci. 2021, 1486, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Shanbehzadeh, S.; Tavahomi, M.; Zanjari, N.; Ebrahimi-Takamjani, I.; Amiri-arimi, S. Physical and Mental Health Complications Post-COVID-19: Scoping Review. J. Psychosom. Res. 2021, 147, 110525. [Google Scholar] [CrossRef] [PubMed]
- Matalon, N.; Dorman-Ilan, S.; Hasson-Ohayon, I.; Hertz-Palmor, N.; Shani, S.; Basel, D.; Gross, R.; Chen, W.; Abramovich, A.; Afek, A.; et al. Trajectories of Post-Traumatic Stress Symptoms, Anxiety, and Depression in Hospitalized COVID-19 Patients: A One-Month Follow-Up. J. Psychosom. Res. 2021, 143, 110399. [Google Scholar] [CrossRef] [PubMed]


| Group-1 (Without Invasive Ventilation) | Group-2 (With Invasive Ventilation) | Effect Size * | p§ 0.05 | |
|---|---|---|---|---|
| Median (min to max) | Median (min to max) | |||
| Sociodemographic and Clinical Data | ||||
| Proportions (%) | 18 (48.60) | 19 (51.40) | ||
| Age (years) | 67.5 (55 to 88) | 63 (29 to 78) | 0.25 (−0.11 to 0.61) | 0.06 |
| Sex | ||||
| Female | 3 (16.70) | 3 (15.80) | 0.88 (−30.37 to 32.12) | 0.64 |
| Male | 15 (83.30) | 16 (84.20) | ||
| Educational level | 10.5 (3 to 18) | 13 (5 to 18) | 0.18 (−0.18 to 0.49) | 0.86 |
| BMI (Kg/m2) | 27.8 (22.3 to 33.8) | 28.05 (22.8 to 37.1) | 0.01 (−0.43 to 0.15) | 0.50 |
| Smoking status | ||||
| Ex-smokers | 8 (44.40) | 8 (42.10) | 2.30 (−13.26 to 7.60) | 0.57 |
| Current smokers | 10 (55.60) | 11 (57.90) | ||
| Time of positivity (days) | 32 (12 to 62) | 40 (5 to 59) | 0.24 (−0.13 to 0.61) | 0.93 |
| COPD | 2 (11.10) | 2 (10.50) | −0.58 (−26.91 to 25.74) | 0.71 |
| Carcinoma | 1 (5.60) | 4 (21.10) | 15.50 (−12.32 to 43.31) | 0.18 |
| Cardiovascular diseases | 8 (44.40) | 5 (26.30) | −18.13 (−57.97 to 21.71) | 0.93 |
| Chronic kidney disease | 6 (33.30) | 3 (15.80) | −17.54 (−53.37 to 18.38) | 0.95 |
| Hypertension | 11 (61.10) | 10 (52.60) | −8.48 (−50.27 to 33.31) | 0.80 |
| Obesity | 8 (44.40) | 5 (26.30) | −18.13 (−57.97 to 21.71) | 0.93 |
| Apneas | 1 (5.60) | 2 (10.50) | 4.97 (−17.88 to 27.82) | 0.53 |
| Diabetes | 9 (50.00) | 5 (26.30) | −23.68 (−63.67 to 16.30) | 0.96 |
| Pneumological and Motor Evaluation | ||||
| FEV1 % | 84 (41 to 102) | 75 (57 to 138) | 0.18 (−0.25 to 0.55) | 0.17 |
| FVC % | 76 (34 to 100) | 67 (40 to 129) | 0.16 (−0.24 to 0.49) | 0.21 |
| FEV1/FVC ratio | 1.08 (0.91 to 1.29) | 1.07 (0.92 to 1.17) | 0.06 (−0.36 to 0.29) | 0.37 |
| DLCO % | 55 (34 to 81) | 48 (1 to 63) | 0.24 (−0.19 to 0.64) | 0.12 |
| HCO3− | 26 (24 to 49) | 25.5 (22 to 30) | 0.02 (−0.47 to 0.20) | 0.54 |
| paCO2 | 36.5 (25 to 71) | 37 (30 to 46) | 0.05 (−0.37 to 0.23) | 0.60 |
| pH | 7.44 (7.39 to 7.49) | 7.44 (7.36 to 7.49) | 0.20 (−0.20 to 0.56) | 0.87 |
| pO2 | 80 (54 to 126) | 70 (44 to 81) | 0.43 (0.05 to 0.80) | <0.01 |
| Spirometry pattern | 53% | 75% | 21.67 (−13.5 to 56.8) | 0.28 |
| Theoretical distance % | 39.5 (12 to 66) | 33.5 (21 to 52) | 0.11 (−0.14 to 0.67) | 0.92 |
| Distance meters | 185 (60 to 385) | 200 (120 to 240) | 0.03 (−0.37 to 0.23) | 0.59 |
| Barthel Scale | 50.50 (13 to 100) | 41 (5 to 60) | 0.34 (−0.13 to 0.81) | 0.20 |
| Heart rate (rest) | 90 (58 to 123) | 101 (72 to 120) | 0.21 (−0.20 to 0.58) | 0.87 |
| Heart rate (maximum) | 106.5 (88 to 124) | 112 (98 to 139) | 0.23 (−0.17 to 0.61) | 0.89 |
| Neuromuscular dysfunction | 3 (27.30) | 7 (53.80) | 26.57 (−23.07 to 76.22) | 0.18 |
| Group-1 (Without Invasive Ventilation) | Group-2 (With Invasive Ventilation) | |||||
|---|---|---|---|---|---|---|
| Median (Range) | Median (Range) | Effect Size * | p (FDR) | Coefficients (95%) ** | Stand. Error | |
| Neuropsychological data | ||||||
| MMSE | 26.5 (20 to 30) | 29 (19 to 30) | 0.29 (−0.01 to 0.58) | 0.25 | −1.01 (−2.46 to 0.43) | 0.74 |
| FAB | 10.50 (5 to 17) | 14 (3 to 16) | 0.39 (0.10 to 0.70) | 0.02 | −1.67 (−3.07 to −0.27) | 0.71 |
| HADS Anxiety | 8.5 (0 to 17) | 7 (4 to 15) | 0.06 (−0.23 to 0.18) | 0.72 | −0.90 (−3.91 to 2.11) | 1.53 |
| HADS Depression | 6 (0 to 15) | 6 (2 to 16) | 0.03 (−0.28 to 0.12) | 0.89 | −1.13 (−3.93 to 1.70) | 1.44 |
| R−BANS Total Score | 74 (51 to 126) | 87 (71 to 106) | 0.28 (−0.02 to 0.57) | 0.09 | −4.34 (−10.89 to 2.21) | 3.34 |
| R−BANS Immediate Memory | 81 (62 to 122) | 93 (59 to 109) | 0.32 (0.03 to 0.62) | 0.04 | −8.33 (−16.99 to 0.19) | 4.38 |
| Story memory | 14.5 (5 to 20) | 17 (1 to 24) | 0.25 (−0.03 to 0.53) | 0.10 | −1.27 (−3.97 to 1.42) | 1.37 |
| List learning | 21 (15 to 35) | 25 (17 to 34) | 0.30 (−0.01 to 0.60) | 0.14 | −2.71 (−5.39 to −0.03) | 1.36 |
| R−BANS Delayed Memory | 86 (63 to 146) | 99 (79 to 128) | 0.18 (0.07 to 0.63) | 0.03 | −10.61 (−21.21 to −0.01) | 5.40 |
| Story Recall | 6.5 (0 to 11) | 8 (3 to 12) | 0.31 (0.02 to 0.60) | 0.04 | −1.01 (−2.71 to 0.68) | 0.86 |
| List Recall | 4 (0 to 9) | 6 (0 to 8) | 0.23 (−0.04 to 0.50) | 0.14 | −1.10 (−3.05 to 0.83) | 0.99 |
| List Recognition | 17 (10 to 20) | 18 (13 to 20) | 0.23 (−0.05 to 0.51) | 0.06 | −0.59 (−1.84 to 0.65) | 0.63 |
| Figure Recall | 10 (5 to 20) | 13 (0 to 20) | 0.38 (0.10 to 0.69) | 0.05 | −2.04 (−4.52 to 0.43) | 1.26 |
| R−BANS Attention | 77.5 (45 to 133) | 85 (65 to 120) | 0.18 (−0.08 to 0.42) | 0.25 | 36.45 (−14.20 to 87.11) | 6.35 |
| Coding | 20 (6 to 55) | 33 (0 to 51) | 0.32 (0.03 to 0.62) | 0.05 | −6.80 (−12.25 to −1.34) | 2.78 |
| Digit Span | 7 (4 to 12) | 8 (5 to 13) | 0.09 (−0.19 to 0.26) | 0.46 | −0.34 (−1.67 to 0.98) | 0.67 |
| R−BANS Language | 79.5 (57 to 103) | 77 (69 to 111) | 0.02 (−0.29 to 0.09) | 0.92 | 3.25 (−5.69 to 7.05) | 3.25 |
| Naming | 10 (8 to 10) | 10 (9 to 10) | 0.04 (−0.25 to 0.15) | 0.45 | 10.00 (−503.23 to 523.23) | 261.85 |
| Semantic Fluency | 14.5 (6 to 26) | 16 (7 to 21) | 0.10 (−0.16 to 0.28) | 0.53 | 0.01 (−3.07 to 3.07) | 1.56 |
| R−BANS Visuospatial/Construction | 88 (58 to 120) | 99 (62 to 123) | 0.16 (−0.12 to 0.39) | 0.33 | −4.07 (−16.34 to 8.20) | 6.26 |
| Figure Copy | 14.5 (10 to 20) | 18 (0 to 20) | 0.20 (−0.07 to 0.47) | 0.22 | −0.91 (−3.57 to 1.74) | 1.35 |
| Line Orientation | 14.5 (3 to 18) | 13 (6 to 20) | 0.12 (−0.15 to 0.31) | 0.29 | −0.04 (−3.00 to 2.91) | 1.50 |
| Pneumological and Motor data | ||||||
| Theoretical distance (%) | 39.5 (12 to 66) | 33.5 (21 to 52) | 0.11 (−0.14 to 0.67) | 0.15 | 0.30 (−17.76 to 18.37) | 9.21 |
| Distance Meters | 185 (60 to 385) | 200 (120 to 240) | 0.03 (−0.31 to 0.16) | 0.84 | −5.23 (−64.00 to 53.53) | 29.89 |
| Barthel Scale | 50.50 (13 to 100) | 41 (5 to 60) | 0.34 (−0.02 to 0.70) | 0.15 | 15.22 (−9.13 to 39.59) | 15.22 |
| FEV (%) | 84 (41 to 102) | 75 (57 to 138) | 0.18 (−0.13 to 0.46) | 0.35 | 2.91 (−6.19 to 12.02) | 4.64 |
| FVC (%) | 76 (34 to 100) | 67 (40 to 129) | 0.16 (−0.16 to 0.40) | 0.42 | 3.35 (−9.89 to 16.61) | 0.38 |
| DLCO (%) | 55 (34 to 81) | 48 (1 to 63) | 0.24 (−0.11 to 0.55) | 0.24 | 4.43 (−7.83 to 16.70) | 6.26 |
| HCO3− | 26 (24 to 49) | 25.5 (22 to 30) | 0.02 (−0.39 to 0.12) | 0.95 | −0.73 (−3.57 to 2.09) | 1.44 |
| paCO2 | 36.5 (25 to 71) | 37 (30 to 46) | 0.05 (−0.29 to 0.16) | 0.81 | −0.33 (−3.84 to 3.16) | 1.78 |
| pO2 | 80 (54 to 126) | 70 (44 to 81) | 0.43 (0.14 to 0.73) | 0.01 | 13.02 (3.31 to 22.74) | 4.95 |
| pH | 7.44 (7.39 to 7.49) | 7.44 (7.36 to 7.49) | 0.20 (−0.10 to 0.48) | 0.27 | −0.02 (−0.04 to 0.01) | 0.01 |
| Group 1 | Group 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Wilcoxon’s Effect Size | p (FDR) | Before | After | Wilcoxon’s Effect Size | p (FDR) | |
| Proportions (%) | 10 (50.00) | 10 (50.00) | 15 (50.00) | 15 (50.00) | ||||
| Neuropsychological Assessment | ||||||||
| HADS/A | 6 (2 to 15) | 6 (2 to 16) | 0.02 (−0.58 to 0.13) | 0.35 | 8 (4 to 15) | 6 (2 to 10) | 0.67 (0.43 to 0.96) | 0.02 |
| HADS/D | 5.5 (2 to 15) | 5.5 (1 to 11) | 0.58 (0.13 to 1.04) | 0.05 | 6 (2 to 16) | 4 (1 to 10) | 0.58 (0.25 to 0.92) | 0.05 |
| R−BANS Total Score | 78 (63 to 107) | 77 (66 to 108) | 0.40 (−0.04 to 0.84) | 0.24 | 84 (71 to 106) | 89 (71 to 121) | 0.59 (0.26 to 0.94) | 0.04 |
| R−BANS Immediate Memory | 79.5 (62 to 109) | 88 (78 to 114) | 0.80 (0.62 to 1.03) | 0.04 | 93 (59 to 109) | 97 (76 to 115) | 0.44 (0.05 to 0.83) | 0.04 |
| Story Memory | 14.5 (10 to 20) | 16 (8 to 21) | 0.14 (−0.39 to 0.38) | 0.34 | 17 (1 to 24) | 18 (15 to 23) | 0.20 (−0.20 to 0.49) | 0.34 |
| List Learning | 19.5 (17 to 28) | 25.5 (18 to 31) | 0.72 (0.43 to 1.06) | 0.04 | 25 (17 to 34) | 28 (15 to 35) | 0.42 (−0.04 to 0.86) | 0.44 |
| R−BANS Delayed Memory | 92.5 (63 to 109) | 102 (72 to 119) | 0.62 (0.22 to 1.02) | 0.02 | 95 (79 to 128) | 101 (83 to 128) | 0.58 (0.22 to 0.95) | 0.02 |
| Story Recall | 7.5 (6 to 11) | 8 (3 to 12) | 0.26 (−0.23 to 0.61) | 0.94 | 9 (3 to 12) | 10 (5 to 11) | 0.31 (−0.09 to 0.68) | 0.46 |
| List Recall | 4 (0 to 7) | 4.5 (0 to 9) | 0.52 (0.06 to 0.97) | 0.32 | 6 (0 to 8) | 6 (3 to 9) | 0.48 (0.12 to 0.86) | 0.02 |
| List Recognition | 17.5 (15 to 19) | 18 (10 to 20) | 0.14 (−0.39 to 0.40) | 0.23 | 18 (13 to 20) | 19 (16 to 20) | 0.62 (0.31 to 0.95) | 0.02 |
| Figure Recall | 11 (6 to 13) | 14 (10 to 17) | 0.76 (0.52 to 1.04) | 0.02 | 13 (0 to 20) | 13 (8 to 20) | 0.28 (−0.11 to 0.62) | 0.34 |
| R−BANS Attention | 80 (62 to 108) | 81 (62 to 115) | 0.42 (−0.09 to 0.9) | 0.23 | 85 (70 to 120) | 96 (72 to 132) | 0.65 (0.36 to 0.94) | 0.02 |
| Coding | 20 (16 to 44) | 28.5 (13 to 41) | 0.57 (0.18 to 1.01) | 0.02 | 33 (0 to 50) | 39 (15 to 54) | 0.43 (0.03 to 0.84) | 0.02 |
| Digit Span | 7 (6 to 11) | 7 (6 to 13) | 0.01 (−0.50 to 1.50) | 0.34 | 8 (5 to 13) | 10 (6 to 12) | 0.25 (−0.18 to 0.60) | 0.34 |
| R−BANS Language | 79.5 (61 to 89) | 80 (76 to 90) | 0.11 (−0.43 to 0.33) | 0.76 | 78 (69 to 111) | 80 (69 to 108) | 0.20 (−0.21 to 0.50) | 0.76 |
| Naming | 10 (8 to 10) | 10 (10 to 10) | 0.01 (−0.50 to 1.50) | 0.89 | 10 (9 to 10) | 10 (9 to 10) | 0.01 (−0.50 to 1.50) | 0.89 |
| Semantic Fluency | 14 (6 to 21) | 13.5 (6 to 22) | 0.40 (−0.11 to 0.86) | 0.85 | 16 (10 to 21) | 14 (8 to 28) | 0.18 (−0.25 to 0.47) | 0.85 |
| R−BANS Visuospatial/Construction | 94 (58 to 119) | 88.5 (75 to 106) | 0.11 (−0.41 to 0.32) | 0.73 | 98 (62 to 118) | 93 (64 to 112) | 0.11 (−0.34 to 0.32) | 0.73 |
| Figure Copy | 15 (10 to 19) | 13.5 (7 to 19) | 0.42 (−0.06 to 0.86) | 0.98 | 18 (0 to 20) | 16 (2 to 19) | 0.19 (−0.24 to 0.48) | 0.98 |
| Line Orientation | 16.5 (3 to 18) | 15 (12 to 18) | 0.22 (−0.28 to 0.56) | 0.45 | 15 (6 to 20) | 17 (11 to 19) | 0.26 (−0.14 to 0.60) | 0.45 |
| Pneumological and Motor Evaluations | ||||||||
| FEV1 % | 89.5 (8) | 77 (25.25) | 0.51 (0.05 to 0.98) | 0.03 | 90 (11.5) | 70 (11.5) | 0.85 (0.79 to 0.94) | 0.03 |
| FVC % | 83 (10) | 65 (23.75) | 0.51 (0.05 to 0.99) | 0.05 | 85 (7.5) | 64 (18) | 0.60 (0.29 to 0.96) | 0.04 |
| FEV1/FVC ratio | 67 (232) | 231 (90.75) | 0.40 (−0.11 to 0.86) | 0.79 | 33 (284) | 222 (53) | 0.60 (0.28 to 0.94) | 0.08 |
| DLCO % | 48 (23) | 46.5 (18.75) | 0.14 (−0.41 to 0.39) | 0.54 | 52 (24) | 40 (11) | 0.59 (0.24 to 0.97) | 0.02 |
| HCO3− | 25 (1.75) | 26 (1.88) | 0.06 (−0.51 to 0.23) | 0.83 | 25 (4) | 26 (3) | 0.57 (0.25 to 0.93) | 0.04 |
| paCO2 | 38 (3.25) | 39 (6.5) | 0.17 (−0.38 to 0.47) | 0.84 | 38 (4.5) | 38 (3.5) | 0.31 (−0.09 to 0.70) | 0.84 |
| pH | 7.44 (0.03) | 7.43 (0.04) | 0.65 (0.28 to 1.03) | 0.04 | 7.45 (365.82) | 7.48 (730.06) | 0.36 (−0.05 to 0.75) | 0.56 |
| pO2 | 74 (5.25) | 77 (14.75) | 0.32 (−0.13 to 0.72) | 0.98 | 76 (13.5) | 70 (16.5) | 0.38 (−0.01 to 0.79) | 0.68 |
| Theoretical distance% | 511 (72.5) | 545 (57.5) | 0.11 (−0.13 to 0.80) | 0.89 | 510 (76.5) | 510 (74) | − | 0.89 |
| Distance meters | 205 (75) | 150 (105) | 0.46 (−0.04 to 0.93) | 0.45 | 240 (35) | 180 (35) | 0.70 (0.25 to 0.92) | 0.04 |
| Barthel Scale | 27.5 (20.75) | 57.5 (18.5) | 0.82 (0.65 to 1.01) | 0.02 | 12 (17.5) | 52 (12) | 0.88 (0.65 to 1.01) | 0.02 |
| Heart rate (rest) | 92.5 (26.25) | 90 (14) | 0.18 (−0.31 to 0.46) | 0.44 | 95 (3) | 85 (13.5) | 0.39 (−0.07 to 0.81) | 0.44 |
| Heart rate (maximum) | 112.5 (14.75) | 104 (15.25) | 0.65 (0.23 to 1.06) | 0.65 | 109 (1.5) | 101.5 (11.5) | 0.31 (−0.16 to 0.72) | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lagravinese, G.; Castellana, G.; Castellana, F.; Genco, M.; Petrelli, R.; Ruccia, M.; Aliani, M.; Carone, M.; Sardone, R.; Battista, P. Cognitive Deficits among Individuals Admitted to a Post-Acute Pneumological Rehabilitation Unit in Southern Italy after COVID-19 Infection. Brain Sci. 2023, 13, 84. https://doi.org/10.3390/brainsci13010084
Lagravinese G, Castellana G, Castellana F, Genco M, Petrelli R, Ruccia M, Aliani M, Carone M, Sardone R, Battista P. Cognitive Deficits among Individuals Admitted to a Post-Acute Pneumological Rehabilitation Unit in Southern Italy after COVID-19 Infection. Brain Sciences. 2023; 13(1):84. https://doi.org/10.3390/brainsci13010084
Chicago/Turabian StyleLagravinese, Gianvito, Giorgio Castellana, Fabio Castellana, Maddalena Genco, Rita Petrelli, Maria Ruccia, Maria Aliani, Mauro Carone, Rodolfo Sardone, and Petronilla Battista. 2023. "Cognitive Deficits among Individuals Admitted to a Post-Acute Pneumological Rehabilitation Unit in Southern Italy after COVID-19 Infection" Brain Sciences 13, no. 1: 84. https://doi.org/10.3390/brainsci13010084
APA StyleLagravinese, G., Castellana, G., Castellana, F., Genco, M., Petrelli, R., Ruccia, M., Aliani, M., Carone, M., Sardone, R., & Battista, P. (2023). Cognitive Deficits among Individuals Admitted to a Post-Acute Pneumological Rehabilitation Unit in Southern Italy after COVID-19 Infection. Brain Sciences, 13(1), 84. https://doi.org/10.3390/brainsci13010084