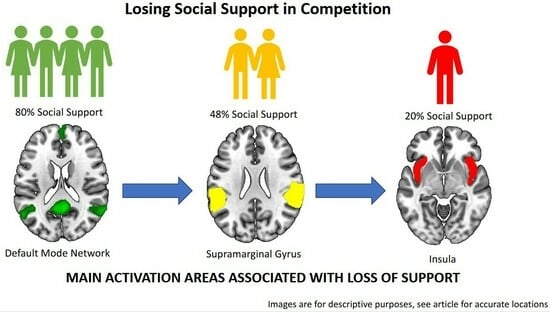
Gradual Loss of Social Group Support during Competition Activates Anterior TPJ and Insula but Deactivates Default Mode Network
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Model and Subject Details
Participants
2.2. Method Details
2.2.1. Psychometric Assessment
2.2.2. The Game
2.2.3. Stimuli and Apparatus
2.2.4. The fMRI Task Protocol
2.3. Quantification and Statistical Analysis
2.3.1. Behavioral Analysis
2.3.2. fMRI Image Acquisition
2.3.3. Preprocessing
2.3.4. Univariate Analyses
2.3.5. Contrasts
2.3.6. Beta Values
3. Results
3.1. Psychometric Results
3.2. fMRI Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phan, T.V.; Morris, R.; Black, M.E.; Do, T.K.; Lin, K.-C.; Nagy, K.; Sturm, J.C.; Bos, J.; Austin, R.H. Bacterial Route Finding and Collective Escape in Mazes and Fractals. Phys. Rev. X 2020, 10, 31017. [Google Scholar] [CrossRef]
- Müller, A.E.; Thalmann, U. Origin and evolution of primate social organisation: A reconstruction. Biol. Rev. 2000, 75, 405–435. [Google Scholar] [CrossRef]
- Shultz, S.; Opie, C.; Atkinson, Q.D. Stepwise evolution of stable sociality in primates. Nature 2011, 479, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Gilovich, T. The Dark Side of Self- and Social Perception: Black Uniforms and Aggression in Professional Sports. J. Pers. Soc. Psychol. 1988, 54, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Cikara, M.; Van Bavel, J.J. The Neuroscience of Intergroup Relations: An Integrative Review. Perspect. Psychol. Sci. 2014, 9, 245–274. [Google Scholar] [CrossRef]
- Tajfel, H.; Turner, J. An integrative theory of intergroup conflict. In The Social Psychology of Intergroup Relations; Austin, W.G., Worchel, S., Eds.; Brooks/Cole: Monterey, CA, USA, 1979; pp. 33–37. [Google Scholar]
- Reicher, S.D.; Spears, R.; Postmes, T. A Social Identity Model of Deindividuation Phenomena. Eur. Rev. Soc. Psychol. 1995, 6, 161–198. [Google Scholar] [CrossRef]
- Molenberghs, P. Neuroscience and Biobehavioral Reviews The neuroscience of in-group bias. Neurosci. Biobehav. Rev. 2013, 37, 1530–1536. [Google Scholar] [CrossRef]
- Knowles, M.L.; Gardner, W.L. Benefits of membership: The activation and amplification of group identities in response to social rejection. Personal. Soc. Psychol. Bull. 2008, 34, 1200–1213. [Google Scholar] [CrossRef]
- Williams, K.D. Ostracism: The Kiss of Social Death. Soc. Pers. Psychol. Compass 2007, 1, 236–247. [Google Scholar] [CrossRef]
- Hawkley, L.C.; Cacioppo, J.T. Loneliness matters: A theoretical and empirical review of consequences and mechanisms. Ann. Behav. Med. 2010, 40, 218–227. [Google Scholar] [CrossRef]
- Cacioppo, S.; Cacioppo, J.T. Decoding the invisible forces of social connections. Front. Integr. Neurosci. 2012, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cheng, J.; Iyer, S.; Naaman, M. When do people trust their social groups? In Proceedings of the 2019 CHI Conference on Human Factors in Computing Systems, Glasgow, UK, 4–9 May 2019. [Google Scholar] [CrossRef]
- Tranel, D.; Cooper, G.; Rodnitzky, R.L. Higher Brain Functions. In Neuroscience in Medicine; Conn, P.M., Ed.; Humana Press: Totowa, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Molnar-Szakacs, I.; Uddin, L.Q. Self-processing and the default mode network: Interactions with the mirror neuron system. Front. Hum. Neurosci. 2013, 7, 571. [Google Scholar] [CrossRef]
- Kernbach, J.M.; Yeo, B.T.T.; Smallwood, J.; Margulies, D.S.; de Schotten, M.T.; Walter, H.; Sabuncu, M.R.; Holmes, A.J.; Gramfort, A.; Varoquaux, G.; et al. Subspecialization within default mode nodes characterized in 10,000 UK Biobank participants. Proc. Natl. Acad. Sci. USA 2018, 115, 12295–12300. [Google Scholar] [CrossRef] [PubMed]
- Yeo, T.B.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [PubMed]
- Christoff, K.; Irving, Z.C.; Fox, K.C.R.; Spreng, R.N.; Andrews-Hanna, J.R. Mind-wandering as spontaneous thought: A dynamic framework. Nat. Rev. Neurosci. 2016, 17, 718–731. [Google Scholar] [CrossRef]
- Brewer, J.A.; Garrison, K.A.; Whitfield-Gabrieli, S. What about the ‘self’ is processed in the posterior cingulate cortex? Front. Hum. Neurosci. 2013, 7, 647. [Google Scholar]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Northoff, G. Self and brain: What is self-related processing? Trends Cogn. Sci. 2011, 15, 186–187. [Google Scholar] [CrossRef]
- Saxe, R. Uniquely human social cognition. Curr. Opin. Neurobiol. 2006, 16, 235–239. [Google Scholar] [CrossRef]
- Cikara, M.; Van Bavel, J.J.; Ingbretsen, Z.A.; Lau, T. Decoding ‘Us’ and ‘Them’: Neural representations of generalized group concepts. J. Exp. Psychol. Gen. 2017, 146, 621–631. [Google Scholar] [CrossRef]
- Lieberman, M.D.; Straccia, M.A.; Meyer, M.L.; Du, M.; Tan, K.M. Social, self, (situational), and affective processes in medial prefrontal cortex (MPFC): Causal, multivariate, and reverse inference evidence. Neurosci. Biobehav. Rev. 2019, 99, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Volz, K.G.; Kessler, T.; von Cramon, D.Y. In-group as part of the self: In-group favoritism is mediated by medial prefrontal cortex activation. Soc. Neurosci. 2009, 4, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.J.; Schaer, M.; Debbané, M. Degrees of separation: A quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci. Biobehav. Rev. 2012, 36, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Northoff, G. How is our self related to midline regions and the default-mode network? Neuroimage 2011, 57, 1221–1233. [Google Scholar] [CrossRef]
- Amodio, D.M.; Frith, C.D. Meeting of minds: The medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006, 7, 268–277. [Google Scholar] [CrossRef]
- Eisenberger, N.I. The Neural Bases of Social Pain: Evidence for Shared Representations with Physical Pain. Psychosom. Med. 2012, 74, 126–135. [Google Scholar] [CrossRef]
- Eisenberger, N.I.; Lieberman, M.D.; Williams, K.D. Does rejection hurt? An FMRI study of social exclusion. Science 2003, 302, 290–292. [Google Scholar] [CrossRef]
- Mwilambwe-Tshilobo, L.; Spreng, R.N. Social exclusion reliably engages the default network: A meta-analysis of Cyberball. Neuroimage 2021, 227, 117666. [Google Scholar] [CrossRef]
- Redcay, E.; Dodell-Feder, D.; Pearrow, M.J.; Mavros, P.L.; Kleiner, M.; Gabrieli, J.D.; Saxe, R. Live face-to-face interaction during fMRI: A new tool for social cognitive neuroscience. Neuroimage 2010, 50, 1639–1647. [Google Scholar] [CrossRef]
- Coria-Avila, G.A.; Manzo, J.; Garcia, L.I.; Carrillo, P.; Miquel, M.; Pfaus, J.G. Neurobiology of social attachments. Neurosci. Biobehav. Rev. 2014, 43, 173–182. [Google Scholar] [CrossRef]
- Feldman, R. The Neurobiology of Human Attachments. Trends Cogn. Sci. 2017, 21, 80–99. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M. Conceiving the Self; Basic Books: New York, NY, USA, 1979. [Google Scholar]
- Rytilä-Manninen, M.; Fröjd, S.; Haravuori, H.; Lindberg, N.; Marttunen, M.; Kettunen, K.; Therman, S. Psychometric properties of the Symptom Checklist-90 in adolescent psychiatric inpatients and age- and gender-matched community youth. Child Adolesc. Psychiatry Ment. Health 2016, 10, 23. [Google Scholar] [CrossRef]
- Jamieson, J.P.; Harkins, S.G.; Williams, K.D. Need threat can motivate performance after ostracism. Pers. Soc. Psychol. Bull. 2010, 36, 690–702. [Google Scholar] [CrossRef]
- Dixon, M.L.; Andrews-Hanna, J.R.; Spreng, R.N.; Irving, Z.C.; Mills, C.; Girn, M.; Christoff, K. Interactions between the default network and dorsal attention network vary across default subsystems, time, and cognitive states. Neuroimage 2017, 147, 632–649. [Google Scholar] [CrossRef] [PubMed]
- Dohmatob, E.; Dumas, G.; Bzdok, D. Dark control: The default mode network as a reinforcement learning agent. Hum. Brain Mapp. 2020, 41, 3318–3341. [Google Scholar] [CrossRef] [PubMed]
- Kucyi, A.; Esterman, M.; Riley, C.S.; Valera, E.M. Spontaneous default network activity reflects behavioral variability independent of mind-wandering. Proc. Natl. Acad. Sci. USA 2016, 113, 13899–13904. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Friston, K.J. The default-mode, ego-functions and free-energy: A neurobiological account of Freudian ideas. Brain 2010, 133, 1265–1283. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.F.; Norton, M.I.; Van Horn, J.D.; Wegner, D.M.; Grafton, S.T.; Macrae, C.N. Wandering minds: The default network and stimulus-independent thought. Science 2007, 315, 393–395. [Google Scholar] [CrossRef]
- Buckner, R.L.; DiNicola, L.M. The brain’s default network: Updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 2019, 20, 593–608. [Google Scholar] [CrossRef]
- Spreng, R.N.; Mar, R.A.; Kim, A.S.N. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J. Cogn. Neurosci. 2009, 21, 489–510. [Google Scholar] [CrossRef]
- Suddendorf, T.; Corballis, M.C. The evolution of foresight: What is mental time travel, and is it unique to humans? Behav. Brain Sci. 2007, 30, 299–351. [Google Scholar] [CrossRef] [PubMed]
- Bzdok, D.; Langner, R.; Schilbach, L.; Jakobs, O.; Roski, C.; Caspers, S.; Laird, A.R.; Fox, P.T.; Zilles, K.; Eickhoff, S.B. Characterization of the temporo-parietal junction by combining data-driven parcellation, complementary connectivity analyses, and functional decoding. Neuroimage 2013, 81, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Clemens, B.; Wagels, L.; Bauchmüller, M.; Bergs, R.; Habel, U.; Kohn, N. Alerted default mode: Functional connectivity changes in the aftermath of social stress. Sci. Rep. 2017, 7, 40180. [Google Scholar] [CrossRef] [PubMed]
- Burhanoglu, B.B.; Uslu, O.; Ozkul, B.; Oguz, K.; Eroglu-Koc, S.; Kizilates-Evin, G.; Candemir, C.; Erdogan, Y.; Mull, D.D.; Kitis, O.; et al. Task-Dependent Brain Connectivity Changes in Response to Varying Levels of Social Exclusion. Br. J. Psychiatry (BJPsych). submitted.
- Igelström, K.M.; Graziano, M.S.A. The inferior parietal lobule and temporoparietal junction: A network perspective. Neuropsychologia 2017, 105, 70–83. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Diquattro, N.E.; Sawaki, R.; Geng, J.J. Effective connectivity during feature-based attentional capture: Evidence against the attentional reorienting hypothesis of TPJ. Cereb. Cortex 2014, 24, 3131–3141. [Google Scholar] [CrossRef]
- Tamber-Rosenau, B.J.; Asplund, C.L.; Marois, R. Functional dissociation of the inferior frontal junction from the dorsal attention network in top-down attentional control. J. Neurophysiol. 2018, 120, 2498–2512. [Google Scholar] [CrossRef]
- Vossel, S.; Weidner, R.; Thiel, C.M.; Fink, G.R. What is ‘Odd’ in Posner’s Location-cueing Paradigm? Neural Responses to Unexpected Location and Feature Changes Compared. J. Cogn. Neurosci. 2009, 21, 30–41. [Google Scholar] [CrossRef]
- Bellucci, G.; Feng, C.; Camilleri, J.; Eickhoff, S.B.; Krueger, F. The role of the anterior insula in social norm compliance and enforcement: Evidence from coordinate-based and functional connectivity meta-analyses. Neurosci. Biobehav. Rev. 2018, 92, 378–389. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel-now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Medford, N.; Critchley, H.D. Conjoint activity of anterior insular and anterior cingulate cortex: Awareness and response. Brain Struct. Funct. 2010, 214, 535–549. [Google Scholar] [CrossRef]
- Modinos, G.; Ormel, J.; Aleman, A. Activation of anterior insula during self-reflection. PLoS ONE 2009, 4, e4618. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, G.; Chernyak, S.V.; Goodyear, K.; Eickhoff, S.B.; Krueger, F. Neural signatures of trust in reciprocity: A coordinate-based meta-analysis. Hum. Brain Mapp. 2017, 38, 1233–1248. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.J.; Smith, A.; Dufwenberg, M.; Sanfey, A.G. Triangulating the Neural, Psychological, and Economic Bases of Guilt Aversion. Neuron 2011, 70, 560–572. [Google Scholar] [CrossRef]
- Menon, V. Salience Network. Brain Mapp. An Encycl. Ref. 2015, 2, 597–611. [Google Scholar]
- O’Doherty, J.P. Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Curr. Opin. Neurobiol. 2004, 14, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Pessiglione, M.; Seymour, B.; Flandin, G.; Dolan, R.J.; Frith, C.D. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 2006, 442, 1042–1045. [Google Scholar] [CrossRef]
- Schultz, W.; Dayan, P.; Montague, P.R. A neural substrate of prediction and reward. Science 1997, 275, 1593–1599. [Google Scholar] [CrossRef]
- Wardle, M.C.; De Wit, H.; Penton-Voak, I.; Lewis, G.; Munafò, M.R. Lack of association between COMT and working memory in a population-based cohort of healthy young adults. Neuropsychopharmacology 2013, 38, 1253–1263. [Google Scholar] [CrossRef]
- Rilling, J.K.; Gutman, D.A.; Zeh, T.R.; Pagnoni, G. A Neural Basis for Social Cooperation expertise, information, opportunities, and a host of ma. Neuron 2002, 35, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Rilling, J.K.; Sanfey, A.G.; Aronson, J.A.; Nystrom, L.E.; Cohen, J.D. The neural correlates of theory of mind within interpersonal interactions. Neuroimage 2004, 22, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Fareri, D.S.; Chang, L.J.; Delgado, M.R. Computational substrates of social value in interpersonal collaboration. J. Neurosci. 2015, 35, 8170–8180. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Luo, Y.; Feng, C. Neural signatures of social conformity: A coordinate-based activation likelihood estimation meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 2016, 71, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Sanfey, A.G. Social Decision-Making: Insights from Game Theory and Neuroscience. Science 2007, 318, 598–602. [Google Scholar] [CrossRef]
- de Fockert, J.W.; Rees, G.; Frith, C.D.; Lavie, N. The Role of Working Memory in Visual Selective Attention. Science 2001, 291, 1803–1806. [Google Scholar] [CrossRef]
- Jenkins, A.C. Rethinking Cognitive Load: A Default-Mode Network Perspective. Trends Cogn. Sci. 2019, 23, 531–533. [Google Scholar] [CrossRef]
- Ramot, M.; Fisch, L.; Harel, M.; Kipervasser, S.; Andelman, F.; Neufeld, M.Y.; Kramer, U.; Fried, I.; Malach, R. A widely distributed spectral signature of task-negative electrocorticography responses revealed during a visuomotor task in the human cortex. J. Neurosci. 2012, 32, 10458–10469. [Google Scholar] [CrossRef]



| Area | Cluster Size | p (FWE) | T | x | y | z |
|---|---|---|---|---|---|---|
| Fair Phase > High Social Support | ||||||
| R Supramarginal Gyrus (BA 40) | 37 | 0.002 | 5.9 | 48 | −22 | 47 |
| L Supramarginal Gyrus (BA 40) | 219 | <0.001 | 7.8 | −54 | −25 | 44 |
| R Visual Areas (BA 18) | 240 | <0.001 | 7.1 | 9 | −91 | 20 |
| R Visual Areas (BA 18) | 34 | <0.001 | 6.2 | 21 | −73 | −7 |
| L dlPFC (BA 44) | 8 | 0.016 | 5.4 | −51 | 8 | 26 |
| High Social Support > Fair Phase | ||||||
| PCC (BA 23) | 322 | <0.001 | 8.8 | 3 | −58 | 23 |
| L mPFC (BA 10) | 56 | <0.001 | 8.3 | −6 | 53 | −4 |
| L Angular Gyrus (BA 39) | 84 | <0.001 | 6.9 | −54 | 64 | 29 |
| R Angular Gyrus (BA 39) | 8 | 0.031 | 5.2 | 51 | −58 | 23 |
| R middle temporal cortex (BA 21) | 85 | <0.001 | 8.5 | 57 | −7 | −16 |
| L middle temporal cortex (BA 21) | 6 | 0.043 | 5.3 | −63 | −14 | −4 |
| Area | Cluster Size | p (FWE) | T | x | y | z |
|---|---|---|---|---|---|---|
| Ostracism Phase > High Social Support | ||||||
| L Supramarginal Gyrus (BA 40) | 2384 | <0.001 | 9.6 | −57 | −25 | 41 |
| R Supramarginal Gyrus (BA 40) | 358 | <0.001 | 7.8 | 51 | −19 | 41 |
| R Insula (BA 13) | 98 | <0.001 | 7.5 | 34 | 23 | −1 |
| L Insula (BA 13) | 41 | <0.001 | 6.3 | −33 | 20 | 2 |
| R Visual Areas (BA 18) | 34 | <0.001 | 6.2 | 21 | −73 | −7 |
| L Dorsolateral PFC (BA 44) | 39 | <0.001 | 6.3 | −51 | 8 | 26 |
| R dlPFC (BA10) | 16 | <0.001 | 6.3 | 42 | 47 | 17 |
| L dlPFC (BA10) | 8 | <0.01 | 5.5 | −33 | 47 | 26 |
| R dPC (BA 7) | 21 | 0.002 | 5.9 | 24 | −58 | 50 |
| L Premotor Areas (BA 6) | 17 | 0.004 | 5.8 | −3 | −13 | 50 |
| R Caudate | 17 | 0.003 | 5.8 | 15 | 17 | −7 |
| R Caudate | 8 | 0.006 | 5.6 | 9 | 11 | 5 |
| L Putamen | 7 | 0.005 | 5.8 | −12 | 2 | 5 |
| High Social Support > Ostracism Phase | ||||||
| PCC (BA 23) | 226 | <0.001 | 7.11 | 3 | −55 | 26 |
| L mPFC (BA 10) | 28 | <0.001 | 6.8 | −6 | 56 | −7 |
| L Angular Gyrus (BA 39) | 92 | <0.001 | 6.7 | −43 | 58 | 29 |
| R Angular Gyrus (BA 39) | 24 | 0.013 | 5.2 | 54 | −61 | 23 |
| R middle temporal cortex (BA 21) | 40 | <0.001 | 7.4 | 57 | −7 | −16 |
| L middle temporal cortex (BA 21) | 30 | 0.043 | 6.3 | −57 | −7 | −19 |
| Area | Cluster Size | p (FWE) | T | x | y | z |
|---|---|---|---|---|---|---|
| Fair Phase > Ostracism Phase | ||||||
| ----- | ||||||
| Ostracism Phase > Fair Phase | ||||||
| R Anterior Insula (BA 13) | 39 | <0.001 | 6.4 | 39 | 20 | −1 |
| L Anterior Insula (BA 13) | 5 | 0.004 | 5.7 | −36 | −17 | 5 |
| L Visual Area (BA 19) | 89 | <0.001 | 7.1 | −13 | −85 | 38 |
| L Visual Area (BA 18) | 16 | 0.003 | 5.8 | −15 | −73 | −14 |
| R Visual Area (BA 19) | 18 | 0.004 | 5.7 | 18 | −73 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozkul, B.; Candemir, C.; Oguz, K.; Eroglu-Koc, S.; Kizilates-Evin, G.; Ugurlu, O.; Erdogan, Y.; Mull, D.D.; Eker, M.C.; Kitis, O.; et al. Gradual Loss of Social Group Support during Competition Activates Anterior TPJ and Insula but Deactivates Default Mode Network. Brain Sci. 2023, 13, 1509. https://doi.org/10.3390/brainsci13111509
Ozkul B, Candemir C, Oguz K, Eroglu-Koc S, Kizilates-Evin G, Ugurlu O, Erdogan Y, Mull DD, Eker MC, Kitis O, et al. Gradual Loss of Social Group Support during Competition Activates Anterior TPJ and Insula but Deactivates Default Mode Network. Brain Sciences. 2023; 13(11):1509. https://doi.org/10.3390/brainsci13111509
Chicago/Turabian StyleOzkul, Burcu, Cemre Candemir, Kaya Oguz, Seda Eroglu-Koc, Gozde Kizilates-Evin, Onur Ugurlu, Yigit Erdogan, Defne Dakota Mull, Mehmet Cagdas Eker, Omer Kitis, and et al. 2023. "Gradual Loss of Social Group Support during Competition Activates Anterior TPJ and Insula but Deactivates Default Mode Network" Brain Sciences 13, no. 11: 1509. https://doi.org/10.3390/brainsci13111509
APA StyleOzkul, B., Candemir, C., Oguz, K., Eroglu-Koc, S., Kizilates-Evin, G., Ugurlu, O., Erdogan, Y., Mull, D. D., Eker, M. C., Kitis, O., & Gonul, A. S. (2023). Gradual Loss of Social Group Support during Competition Activates Anterior TPJ and Insula but Deactivates Default Mode Network. Brain Sciences, 13(11), 1509. https://doi.org/10.3390/brainsci13111509