Non-Convulsive Status Epilepticus in Aneurysmal Subarachnoid Hemorrhage: A Prognostic Parameter
Abstract
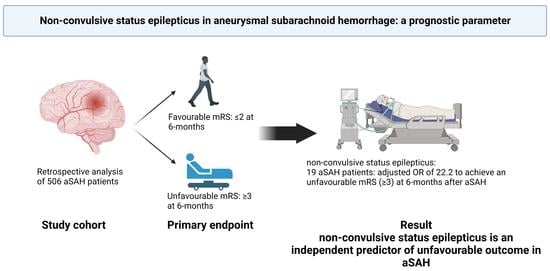
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Management
2.3. Outcome Assessment
2.4. Detection of Epileptic Seizure
2.5. Statistical Analysis
3. Results
3.1. Multivariate Analysis for Outcome
3.2. Outcome According to the Presence of the Seizure
3.3. Fisher and Hunt and Hess Scales
3.4. Clipping vs. Coiling
3.5. Other Complications
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Van Gijn, J.; Kerr, R.S.; Rinkel, G.J. Subarachnoid haemorrhage. Lancet 2007, 369, 306–318. [Google Scholar] [CrossRef]
- Guyo, Y.; Fang, S.; Wang, J.; Zhao, J.; Gai, Y. Continuous EEG detection of DCI and seizures following aSAH: A systematic review. Br. J. Neurosurg. 2020, 34, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Butzkueven, H.; Evans, A.H.; Pitman, A.; Leopold, C.; Jolley, D.J.; Kaye, A.H.; Kilpatrick, C.J.; Davis, S.M. Onset seizures independently predict poor outcome after subarachnoid hemorrhage. Neurology 2000, 55, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Vychopen, M.; Hamed, M.; Bahna, M.; Racz, A.; Ilic, I.; Salemdawod, A.; Schneider, M.; Lehmann, F.; Eichhorn, L.; Bode, C.; et al. A validation Study for SHE Score for Acute Subdural Hematoma in the Elderly. Brain Sci. 2022, 12, 981. [Google Scholar] [CrossRef] [PubMed]
- Kikuta, Y.; Kubota, Y.; Nakamoto, H.; Chernov, M.; Kawamata, T. Nonconvulsive status epilepticus after surgery for ruptured intracranial aneurysms: Incidence, associated factors, and impact on the outcome. Clin. Neurol. Neurosurg. 2021, 200, 106298. [Google Scholar] [CrossRef] [PubMed]
- Bögli, S.Y.; Wang, S.; Romaguera, N.; Schütz, V.; Rafi, O.; Gilone, M.; Keller, E.; Imbach, L.L.; Brandi, G. Impact of Seizures and Status Epilepticus on Outcome in Patients with Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care. 2022, 36, 751–759. [Google Scholar] [CrossRef]
- Vespa, P.M.; Miller, C.; McArthur, D.; Eliseo, M.; Etchepare, M.; Hirt, D.; Glenn, T.C.; Martin, N.; Hovda, D. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit. Care Med. 2007, 35, 2830–2836. [Google Scholar] [CrossRef] [Green Version]
- Mathern, G.W.; Babb, T.L.; Leite, J.P.; Pretorius, K.; Yeoman, K.M.; Kuhlman, P.A. The pathogenic and progressive features of chronic human hippocampal epilepsy. Epilepsy Res. 1996, 26, 151–161. [Google Scholar] [CrossRef]
- Teasdale, G.M.; Drake, C.G.; Hunt, W.; Kassel, N.; Sano, K.; Petruiset, B.; De Villiers, J.C. A universal subarachnoid hemorrhage scale: Report of a committee of the world federation of neurosurgical societies. J. Neurol. Neurosurg. Psychiatry 1998, 51, 1457. [Google Scholar] [CrossRef] [Green Version]
- Schuss, P.; Hadjiathanasiou, A.; Borger, V.; Wispel, C.; Vatter, H.; Guresir, E. Poor-grade aneurysmal subarachnoid hemorrhage: Factors influencing functional outcome-a single center series. World Neurosurg. 2016, 85, 125–129. [Google Scholar] [CrossRef]
- Schuss, P.; Hadjiathanasiou, A.; Brandecker, S.; Guresir, A.; Borger, V.; Wispel, C.; Vatter, H.; Guresir, E. Anticoagulation therapy in patients suffering from aneurysmal subarachnoid hemorrhage: Influence on functional outcome-a single-center series and multivariate analysis. World Neurosurg. 2017, 99, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Guresir, E.; Wispel, C.; Borger, V.; Hadjiathanasiou, A.; Vatter, H.; Schuss, P. Treatment of partially thrombosed intracranial aneurysms: Single-center series and systematic review. World Neurosurg. 2018, 118, e834–e841. [Google Scholar] [CrossRef] [PubMed]
- Schuss, P.; Wispel, C.; Borger, V.; Guresir, A.; Vatter, H.; Guresir, E. Accuracy and safety of ventriculostomy using two different procedures of external ventricular drainage: A single center series. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2018, 79, 206–210. [Google Scholar]
- Raabe, A.; Beck, J.; Keller, M.; Vatter, H.; Zimmermann, M.; Seifert, V. Relative importance of hypertension compared with hypervolemia for increasing cerebral oxygenation in patients with cerebral vasospasm after subarachnoid hemorrhage. J. Neurosrug. 2005, 103, 974–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güresir, E.; Welchowski, T.; Lampmann, T.; Brandecker, S.; Güresir, A.; Wach, J.; Lehmann, F.; Dorn, F.; Velten, M.; Vatter, H. Delayed Cerebral Ischemia after Aneurysmal Subarachnoid Hemorrhage: The Results of Induced Hypertension Only after the IMCVS Trial-A Prospective Cohort Study. J. Clin. Med. 2022, 11, 5850. [Google Scholar] [CrossRef]
- Vergouwen, M.D.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.; et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef] [Green Version]
- Beniczky, S.; Hirsch, L.J.; Kaplan, P.W.; Pressler, R.; Bauer, G.; Aurlien, H.; Brøgger, J.C.; Trinka, E. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia 2013, 54 (Suppl. 6), 28–29. [Google Scholar] [CrossRef]
- Leitinger, M.; Trinka, E.; Zimmermann, G.; Beniczky, S. Salzburg criteria for nonconvulsive status epilepticus: Details matter. Epilepsia 2019, 60, 2334–2336. [Google Scholar] [CrossRef]
- Sánchez Fernández, I.; Goodkin, H.P.; Scott, R.C. Pathophysiology of convulsive status epilepticus. Seizure 2019, 68, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, J.; Johnson, M.A.; McArthur, D.L.; Lutkenhoff, E.S.; Dell’Italia, J.; Real, C.; Shrestha, V.; Spivak, N.M.; Ruiz Tejeda, J.E.; Vespa, P.M.; et al. Sedation-Induced Burst Suppression Predicts Positive Outcome Following Traumatic Brain Injury. Front. Neurol. 2021, 12, 750667. [Google Scholar] [CrossRef] [PubMed]
- Towne, A.R.; Waterhouse, E.J.; Boggs, J.G.; Garnett, L.K.; Brown, A.J.; Smith Jr, J.R.; DeLorenzo, R.J. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology 2000, 54, 340–345. [Google Scholar] [CrossRef]
- Little, A.S.; Kerrigan, J.F.; McDougall, C.G.; Zabramski, J.M.; Albuquerque, F.C.; Nakaji, P.; Spetzler, R. Nonconvulsive status epilepticus in patients suffering spontaneous subarachnoid hemorrhage. J. Neurosurg. 2007, 106, 805–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennis, L.J.; Claassen, J.; Hirsch, L.J.; Emerson, R.G.; Connolly, E.S.; Mayer, S.A. Nonconvulsive status epilepticus after subarachnoid hemorrhage. Neurosurgery 2002, 51, 1136–1144. [Google Scholar] [CrossRef]
- Claassen, J.; Mayer, S.A.; Kowalski, R.G.; Emerson, R.G.; Hirsch, L.J. Detection of electrographic seizures with continuous eeg monitoring in critically ill patients. Neurology 2004, 62, 1743–1748. [Google Scholar] [CrossRef]
- De Marchis, G.M.; Pugin, D.; Meyers, E.; Velasquez, A.; Suwatcharangkoon, S.; Park, S.; Falo, M.C.; Agarwal, S.; Mayer, S.; Michaela Schmidt, J.; et al. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology 2016, 86, 253–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindgren, C.; Nordh, E.; Naredi, S.; Olivecrona, M. Frequency of non-convulsive seizures and non-convulsive status epilepticus in subarachnoid hemorrhage patients in need of controlled ventilation and sedation. Neurocrit. Care. 2012, 17, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Connolly, E.S., Jr.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, L.B.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef] [Green Version]
- Claassen, J.; Peery, S.; Kreiter, K.T.; Hirsch, L.J.; Du, E.Y.; Connolly, E.S.; Mayer, S.A. Predictors and clinical impact of epilepsy after subarachnoid hemorrhage. Neurology 2003, 60, 208–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidech, A.M.; Kreiter, K.T.; Janjua, N.; Ostapkovich, N.; Parra, A.; Commichau, C.; Connolly, E.S.; Mayer, S.A.; Fitzsimmons, B.-F.M. Phenytoin exposure is associated with functional and cognitive disability after subarachnoid hemorrhage. Stroke 2005, 36, 583–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosengart, A.J.; Huo, J.D.; Tolentino, J.; Novakovic, R.L.; Frank, J.I.; Goldenberg, F.D.; Macdonald, R.L. Outcome in patients with subarachnoid hemorrhage treated with antiepileptic drugs. J. Neurosurg. 2007, 107, 253–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marigold, R.; Gunther, A.; Tiwari, D.; Kwan, J. Antiepileptic drugs for the primary and secondary prevention of seizures after subarachnoid haemorrhage. Cochrane Database Syst. Rev. 2013, 2013, CD008710. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Freeman, W.D. The Double-Edged Sword of Seizures and Nonconvulsive Status Epilepticus on Aneurysmal Subarachnoid Hemorrhage Outcomes. Neurocrit. Care. 2022, 36, 699–701. [Google Scholar] [CrossRef] [PubMed]
- Claassen, J.; Albers, D.; Schmidt, J.M.; De Marchis, G.M.; Pugin, D.; Falo, C.M.; Mayer, S.A.; Cremers, S.; Agarwal, S.; Elkind, M.S.; et al. Nonconvulsive seizures in subarachnoid hemorrhage link inflammation and outcome. Ann. Neurol. 2014, 75, 771–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guresir, E.; Lampmann, T.; Bele, S.; Czabanka, M.; Czorlich, P.; Gempt, J.; Goldbrunner, R.; Hurth, H.; Hermann, E.; Jabbarli, R.; et al. Fight INflammation to Improve outcome after aneurysmal Subarachnoid HEmorRhage (FINISHER) trial: Study protocol for a randomized controlled trial. Int. J. Stroke 2022, 17474930221093501. [Google Scholar] [CrossRef] [PubMed]

| No ncSE (n = 487) | ncSE (n = 19) | p Values | |
|---|---|---|---|
| Age (years) | 57 ± 13.7 | 62 ± 10.6 | 0.49 |
| Female (%) | 312 (64) | 14 (74) | 0.47 |
| WFNS score | 3 (IQR 1–5) | 3 (IQR 2–5) | 0.19 |
| GCS on admission | 10 (IQR 3–14) | 10 (IQR 3–14) | 0.84 |
| ICH (%) | 106 (22) | 2 (10) | 0.39 |
| IVH (%) | 158 (32) | 9 (47) | 0.21 |
| Anterior-circulation aneurysm (%) | 409 (84) | 17 (89) | 0.71 |
| Posterior-circulation aneurysm (%) | 78 (16) | 2 (11) | 0.71 |
| Clipping (%) | 226 (47) | 9 (47) | 0.53 |
| Coiling (%) | 231 (47) | 10 (53) | 0.53 |
| No treatment (%) | 30 (6) | 0 (0) | 0.53 |
| Hydrocephalus (%) | 334 (66) | 14 (74) | 0.80 |
| Clinically relevant vasospasm (%) | 227 (47) | 9 (47) | 0.65 |
| DCI (%) | 71 (15) | 5 (26) | 0.18 |
| Favorable outcome (mRS 0–2) (%) | 236 (49) | 1 (5) | 0.001 |
| p Value | Odds Ratio | Confidence Interval | |
|---|---|---|---|
| ncSE | 0.003 | 24.1 | 2.9–195.3 |
| Poor-grade SAH | 0.001 | 14.0 | 8.5–23.1 |
| Age DIND | 0.001 0.003 | 2.8 1.9 | 1.6–4.6 1.2–3.1 |
| No Seizure | Isolated Seizures (n = 487) | ncSE (n = 19) | CSE | |
|---|---|---|---|---|
| Favorable outcome (mRS 0–2) [Percentage deviation] | 274 [+15.4%] | 37 [−39.9%] | 1 [−92.3%] | 0 [N/A] |
| Poor (mRS ≥ 3) [Percentage deviation] | 107 [−26.4%] | 66 [+75%] | 18 [+127.6%] | 3 [N/A] |
| Mortality on discharge day [Percentage deviation] | 97 [+2%] | 21 [−14%] | 7 [+35%] | 3 [N/A] |
| No Seizure | Isolated Seizures (n = 487) | ncSE (n = 19) | p Value | |
|---|---|---|---|---|
| Fisher Grade | 0.58 | |||
| 1 | 20 | 2 | 1 | |
| 2 | 6 | 1 | 1 | |
| 3 | 338 | 98 | 16 | |
| 4 | 16 | 3 | 1 | |
| Hunt and Hess | 0.006 | |||
| 1–2 | 185 | 37 | 4 | |
| 3–5 | 195 | 67 | 15 |
| Anterior Circulation | Posterior Circulation | Isolated Seizure | ncSE | |
|---|---|---|---|---|
| Clipping (n = 235) | 221 | 14 | 58 | 9 |
| Coiling (n = 241) | 186 | 55 | 42 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vychopen, M.; Lampmann, T.; Asoglu, H.; Güresir, A.; Vatter, H.; Wach, J.; Güresir, E. Non-Convulsive Status Epilepticus in Aneurysmal Subarachnoid Hemorrhage: A Prognostic Parameter. Brain Sci. 2023, 13, 184. https://doi.org/10.3390/brainsci13020184
Vychopen M, Lampmann T, Asoglu H, Güresir A, Vatter H, Wach J, Güresir E. Non-Convulsive Status Epilepticus in Aneurysmal Subarachnoid Hemorrhage: A Prognostic Parameter. Brain Sciences. 2023; 13(2):184. https://doi.org/10.3390/brainsci13020184
Chicago/Turabian StyleVychopen, Martin, Tim Lampmann, Harun Asoglu, Agi Güresir, Hartmut Vatter, Johannes Wach, and Erdem Güresir. 2023. "Non-Convulsive Status Epilepticus in Aneurysmal Subarachnoid Hemorrhage: A Prognostic Parameter" Brain Sciences 13, no. 2: 184. https://doi.org/10.3390/brainsci13020184
APA StyleVychopen, M., Lampmann, T., Asoglu, H., Güresir, A., Vatter, H., Wach, J., & Güresir, E. (2023). Non-Convulsive Status Epilepticus in Aneurysmal Subarachnoid Hemorrhage: A Prognostic Parameter. Brain Sciences, 13(2), 184. https://doi.org/10.3390/brainsci13020184